DOI:10.19476/j.ysxb.1004.0609.2018.03.15
锂离子电池正极材料LiMnBO3/C的合成及其电化学性能
陈 伟1,张 华3,张晓萍2,刘洁群2,刘士军1,钟胜奎2
(1. 中南大学 化学化工学院, 长沙 410083;
2. 苏州大学 钢铁学院, 苏州 215021;
3. 上海杉杉科技有限公司, 上海 201209)
摘 要:
以LiOH·H2O、Mn(CH3COO)2·4H2O和H3BO3为原料,聚乙二醇6000(PEG-6000)为碳源,采用喷雾干燥法合成LiMnBO3和LiMnBO3/C正极材料。XRD测试表明,两种样品均为单一的六方晶体结构LiMnBO3(h-LiMnBO3);电化学测试表明,在电压范围1.0~4.8 V内,LiMnBO3在0.5C倍率下的首次放电比容量为63.28 mA·h/g,而LiMnBO3/C的首次放电比容量高达135.21 mA·h/g;循环50次后,两者比容量分别为31.15 mA·h/g和109.69 mA·h/g。碳源的加入有效地提升了LiMnBO3的电化学性能。
关键词:
文章编号:1004-0609(2018)-03-0565-07 中图分类号:TM912 文献标志码:A
自2001年LEGAGNYER等[1]首次报道LiMBO3 (M=Mn、Fe、Co)以来,锂过渡金属硼酸盐材料LiMBO3受到了广泛地关注[2-4]。由于B原子具有高度的亲氧性,可与O原子进行3次或4次配位形成BO3和BO4,三角形的BO3与四面体的BO4能以多种方式形成岛状、链状、环状、层状和三维网状等多种结构类型的硼氧聚阴离子基团,这种结构的多样性使得硼酸盐材料具有多样化的性能,如非线性光学、铁电、压电和半导体行为等特性。锂过渡金属硼酸盐LiMBO3通过三角双金字塔的MO5与BO3平面构成三维(MBO3)nn-框架,使得锂离子能够从材料中反复脱嵌[5-6]。此外,虽然硼元素的电负性小于磷元素的电负性而导致聚阴离子的诱导效应减小,使得硼酸盐材料的嵌脱锂电位比相应的磷酸盐低0.4 V,但与PO43-相比,BO33-具有更小的相对分子质量(58.8 g/mol,远小于95 g/mol),因此,硼酸盐理论能量密度比相应的磷酸盐材料的理论能量密度仍高出10%[7-9]。
在LiMBO3中,由于LiMnBO3具有较高的充放电电压平台,因此受到了更多的关注,LiMnBO3具有两种晶体结构,单斜结构的LiMnBO3(m-LiMnBO3)和六方晶体结构的LiMnBO3(h-LiMnBO3),两种结构的LiMnBO3具有相同的理论比容量(220 mA·h/g),单斜晶体结构的LiMnBO3充放电电压平台为3.7 V,六方晶体结构的为4.1 V[10-14]。然而,LiMnBO3正极材料也存在着一些缺点,对水和氧气非常敏感,常温下接触少量潮湿的空气会导致Mn2+的部分氧化以及晶体结构里锂的缺失,导致电化学性能下降,与LiMnPO4相似,也具有电子电导率和离子电导率低的问题[15]。据此,研究者提出了诸多措施解决上述问题,其中碳包覆是一种有效的方法[16-18]。LI等[19]以LiOH·H2O、MnCO3和H3BO3为原料、抗坏血酸为碳源、在500和750 ℃分别合成出m-LiMnBO3/C和h-LiMnBO3/C),在0.05C倍率下,m-LiMnBO3/C和h-LiMnBO3/C的首次放电比容量分别为107和90.7 mA·h/g,循环40次后,容量保有率仍然达到80.4%和86.5%,显示出较好的循环性能[19]。LEE等[20]以Li2CO3、MnCO3和H3BO3为原料、乙炔黑和蔗糖为碳源分别制备出了LiMnBO3和LiMnBO3/C,在0.05C倍率下LiMnBO3的首次放电比容量只有40 mA·h/g,而以乙炔黑和蔗糖为碳源制备出的LiMnBO3/C首次放电比容量分别为169和135 mA·h/g,包覆碳的LiMnBO3电化学性能得到了明显改善,这是由于碳源的加入在烧结过程中可以有效抑制颗粒的长大,而且分解的残留的碳包覆在材料表面,提高了材料的导电性,从而改善其电化学性能,并且包覆在材料表面的碳层可以有效地隔绝潮湿空气对材料的腐蚀[20]。
本文作者采用聚乙二醇6000(PEG-6000)为碳源合成出了LiMnBO3/C,聚乙二醇作为一种表面活性剂,在形成溶胶的过程中可以大大降低胶粒的表面张力,使得胶粒的粒径变小,在高温烧结的过程中包覆在前躯体表面的PEG裂解成网状结构的碳,抑制了晶体的长大,使得材料的粒径小而且分布均匀,很好地改善了材料的团聚性,包覆在颗粒表面的碳层可以有效地隔绝潮湿空气对LiMnBO3的腐蚀并且大大提高了材料的电子电导率,提高了材料的电化学性能[21-24]。
1 实验
1.1 样品的合成
按摩尔比(1:1:1)称量LiOH·H2O(分析纯,纯度95%)、Mn(CH3COO)2·4H2O(分析纯,纯度99%)和H3BO3(分析纯,纯度 99.5%)溶解在去离子水中,搅拌混合均匀形成溶液。采用喷雾干燥法,进风温度为200 ℃,出风温度为100 ℃,用上述溶液制备前驱体,并将前驱体在120 ℃温度下真空干燥12 h,然后将前驱体置于管式炉中在氩气保护气氛下350 ℃预烧3 h,接着在800 ℃下煅烧10 h,自然冷却得到样品,记为样品LiMnBO3[25-26]。
按摩尔比(1:1:1)称量LiOH·H2O(分析纯,纯度95%)、Mn(CH3COO)2·4H2O(分析纯,纯度99%)和H3BO3(分析纯,纯度99.5%)溶解在去离子水中,搅拌混合均匀形成溶液。称取适量的聚乙二醇-6000 (PEG-6000)溶解在去离子水中,PEG-6000与Mn(CH3COO)2·4H2O的质量比为1:1,然后将上述溶液混合搅拌直至形成溶胶。采用喷雾干燥法,进风温度为200 ℃,出风温度为100 ℃,用上述溶胶制备前驱体,并将前驱体在120 ℃温度下真空干燥12 h,然后将前驱体置于管式炉中在氩气保护气氛下350 ℃预烧3 h,然后在800 ℃下煅烧10 h,自然冷却得到样品,记为样品LiMnBO3/C。
1.2 样品的表征
采用日本Rigaku公司生产的Ultima VI型X射线衍射仪分析样品的物相结构,以Cu Kα1为射线源,扫描范围为10°~90°。采用日本电子株式会社公司的JSM-6380 LV型扫描电子显微镜和美国FEI公司的TecnaiG220型透射电镜观察样品的表面形貌。拉曼测试采用英国Renishaw公司的inVa型拉曼光谱仪。
1.3 电化学测试
将800 ℃合成的材料作为正极活性物质,按活性物质、乙炔黑、PVDF质量比80:10:10均匀混合后调浆涂在铝铂上,放于真空干燥箱中干燥24 h后压片,压片后切成1 cm×1 cm大小的极片,继续放于真空干燥箱内干燥12 h;采用锂片作为负极,1 mol/L LiFP6/EC+DMC(体积比1:1)为电解液,在高纯氩手套箱里组装成实验电池。电池的电化学性能采用新威测试仪测试,充放电电压设定在1.0~4.8 V。本实验中采用CHI660D型电化学工作站测试不同样品的交流阻抗谱,电位振幅为5 mV,频率范围为0.01 Hz~100 kHz。
2 结果与讨论
2.1 X射线衍射分析
图1所示为LiMnBO3和LiMnBO3/C样品的XRD谱。从图1中可以看出,样品具有尖锐的衍射峰,表明样品的结晶度较好,并且样品的特征衍射峰的位置与六方晶体结构LiMnBO3的PDF卡片相一致,在X射线衍射谱上没有观察碳的特征衍射峰,证明PEG-6000分解得到的碳为无定形并且对材料的结构没有影响。为了进一步确定样品的结构信息,本实验中采用Rietveld方法(Fullprof软件)对样品的XRD数据进行了精修。如图2所示,衍射峰的位置和强度与计算所得的匹配性较好,精修结果具有相对较高的可靠性因子,LiMnBO3和LiMnBO3/C样品的可靠性因子(Rw)分别为9.05%和9.20%,两种样品的晶胞参数如表1。碳-硫测试表明LiMnBO3的碳含量为0.2%(质量分数),LiMnBO3/C的碳含量为5.4%。

图1 LiMnBO3和LiMnBO3/C的XRD谱
Fig. 1 XRD patterns of LiMnBO3 and LiMnBO3/C
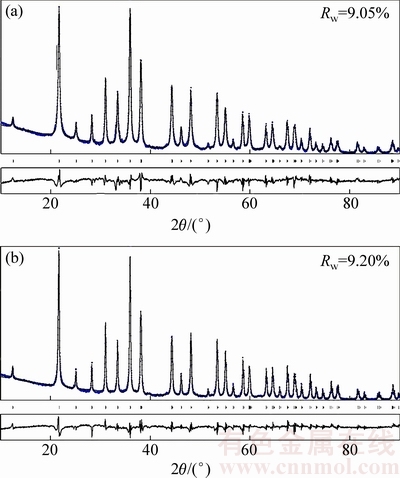
图2 LiMnBO3和LiMnBO3/C的Rietveld精修XRD谱
Fig. 2 Rietveld refinement XRD data of LiMnBO3 (a) and LiMnBO3/C (b)
2.2 形貌分析
图3所示为LiMnBO3和LiMnBO3/C样品在不同放大倍率下的SEM像。从图3(a)和(b)可知,LiMnBO3样品为球形颗粒,粒径分布为0.5~4 μm,颗粒表面比较光滑。从图3(c)和(d)中观察到LiMnBO3/C样品的球形颗粒表面为多孔状,这是由于PEG-6000在高温烧结的过程裂解生成的碳包覆在一次颗粒的表面,并且在烧结的过程中生成的碳可以阻止一次颗粒的长大且小的球形颗粒填充在大颗粒的空隙中,有效提高了材料的振实密度,LiMnBO3和LiMnBO3/C样品的振实密度分别为1.08和1.13 g/cm3。PEG分解所得的碳均匀覆盖在LiMnBO3的表面,形成了三维导电网络,这不仅有效提高了材料的电子电导率,并能显著降低材料电化学反应过程中的极化。
图4所示为LiMnBO3/C样品的TEM像。从图4可知,晶粒通过碳纳米网相连,从图4(a)可以看出,颗粒表面有晶格条纹的晶体区与非晶区界限明显,颗粒表面包覆约3.8 nm的碳层,该碳层不仅可以改善材料颗粒的电子输运能力,提高电子电导率,减弱电池的极化现象,增强材料的电化学性能,还可以调控颗粒生长,得到合适的晶粒尺寸。从图4(b)可知,测试部分的层间距为0.19 nm,与(310)面得d值吻合。这个结果进一步证实了LiMnBO3/C复合材料的形成。
表1 样品结构精修结果
Table 1 Results of structural analysis obtained from X-ray Rietveld refinement
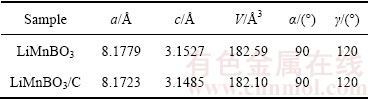
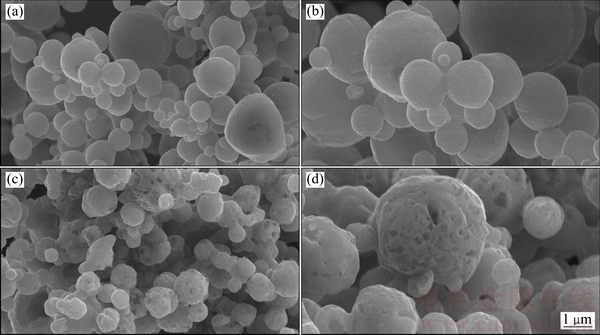
图3 LiMnBO3和LiMnBO3/C的SEM像
Fig. 3 SEM images of LiMnBO3 ((a), (b)) and LiMnBO3/C ((c), (d))
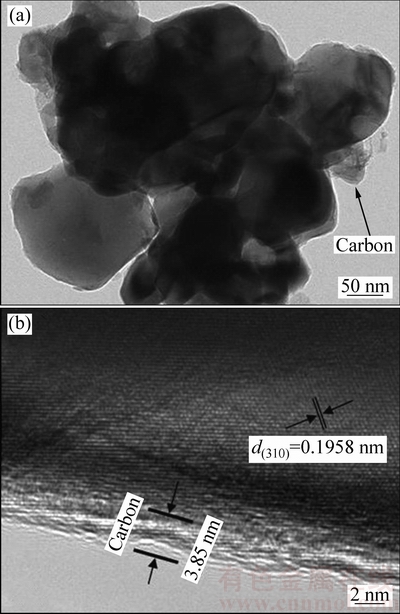
图4 LiMnBO3/C的TEM像
Fig. 4 TEM image (a) and HRTEM image (b) of LiMnBO3/C
为了证实合成的材料实现了原位包覆,本实验中对LiMnBO3/C样品进行了拉曼测试,结果如图5所示。从图5可以观察到,在1355 cm-1(D-band)和1600 cm-1(G-band)处有2个强峰。有机物在高温条件下分解而成的无定形碳有sp3和sp2杂化的原子键构成的,sp3代表了无序的非晶相,sp2代表了有序的石墨相,非晶相的电导性远低于石墨相的。这个实验的结果证实了本实验中合成的产物中确实含有石墨相的碳,石墨化碳的包覆有利于提高材料的导电性,从而提高了电池的性能。
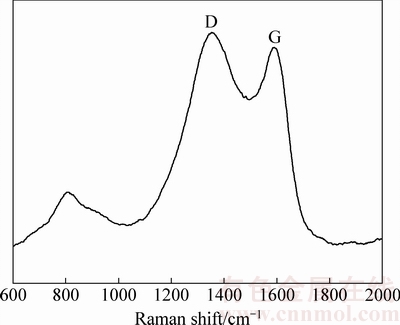
图5 LiMnBO3/C样品的拉曼光谱
Fig. 5 Raman spectrum of LiMnBO3/C
2.3 LiMnBO3/C的电化学性能
图6所示为样品在电压范围1.0~4.8 V内不同倍率下的首次充放电曲线。由图6(a)可知,LiMnBO3样品在0.025C、0.05C、0.1C、0.2C、0.5C和1C倍率下首次放电的比容量分别为168.78、116.95、98.55、84.95、63.27和26.45 mA·h/g,而LiMnBO3/C样品在相同倍率下的首次放电比容量分别高达200.41、172.02、163.24、154.21、135.21和112.03 mA·h/g(见图6(b))。图7所示为样品的循环性能图,LiMnBO3在0.025C、0.05C、0.1C、0.2C、0.5C和1C倍率下循环50次后放电比容量分别为109.54、81.34、77.07、52.91、31.25和22.72 mA·h/g(见图7(a))。LiMnBO3/C样品显示了较好的循环性能,在0.025C、0.05C、0.1C、0.2C、0.5C和1C倍率下循环50次后放电比容量仍然有160.88、144.32、130.21、118.78、109.69和92.70 mA·h/g(见图7(b))。良好的循环性能表明PEG-6000经后续高温煅烧处理后均匀包覆在LiMnBO3颗粒表面,不但可以改善电子在材料颗粒之间的运输能力,减弱电化学极化,同时,有效缩短了锂离子在其内部扩散传输距离,增加了活性物质利用率。并且原位包覆的碳隔绝了潮湿空气对材料的腐蚀并且在在循环过程中电解液进入多孔疏松的碳层,降低了材料在充放电过程中的体积变化率,提高了材料的稳定性。因此,碳包覆有利于提高材料的电化学性能。
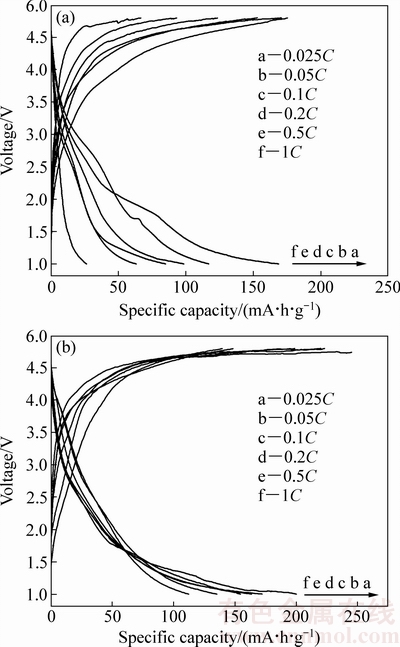
图6 在不同倍率下LiMnBO3和LiMnBO3/C的首次充放电曲线
Fig. 6 Initial charge-discharge curves of LiMnBO3(a) and LiMnBO3/C(b) at different rates

图7 在不同倍率下LiMnBO3和LiMnBO3/C的循环性能图
Fig. 7 Cycle performance of LiMnBO3(a) and LiMnBO3/C(b) at different rates
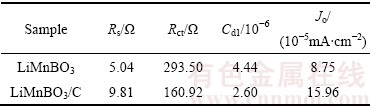
图8所示为LiMnBO3和LiMnBO3/C样品的交流阻抗谱。两条谱线均由一个高频区的半圆和低频区得一条斜线组成,高频区的半圆对应于电荷传输阻抗,低频区的直线代表Li+ 在电极材料中扩散所引起的Warburg阻抗。为了得出更加准确的阻抗参数,用图8的模拟电路对阻抗曲线进行了模拟,电路中Rs代表了电解液电阻;Rct代表了锂离子在电极/电解液界面的电荷转移电阻,CPE表示双电层容;Zw 代表Warburg阻抗,模拟得到的阻抗参数Rs和Rct以及计算得到的双电层电容(Cd1)及交换电流密度(Jo)见表2。
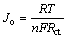 (1)
(1)
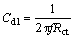 (2)
(2)
式中:R为摩尔气体常数;T为绝对温度;n为转移电子数;F为法拉第常数;Rct为电荷转移电阻;f为频率。
由表2可知,LiMnBO3/C样品的Rct(160.92 Ω)小于LiMnBO3样品的Rct(293.50 Ω),说明碳包覆能提高电解质与氧化物电极界面的电荷传递速率,也使低频区直线的斜率增大,即Zw减小有利于Li+在电极材料中的扩散;双电层电容表示在充放电过程中的电压差,LiMnBO3/C样品的Cd1(2.60×10-6)小于LiMnBO3样品的Cdl(4.44×10-6),说明在充放电过程中电压曲线会更光滑;交换电流密度(Jo)表示平衡电位下电极氧化或还原反应的速率,LiMnBO3/C样品的Jo(15.96×10-5 mA/cm2)大于LiMnBO3样品的Jo(8.75×10-5 mA/cm2),说明碳包覆能提高LiMnBO3电极反应的可逆性,使得电极反应更加容易进行。
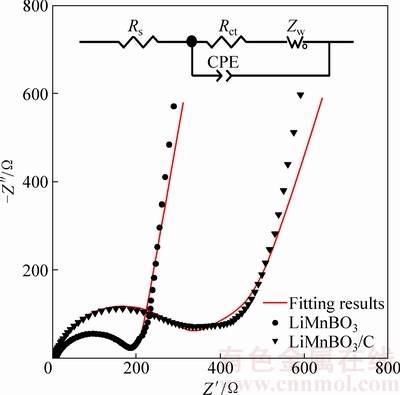
图8 LiMnBO3和LiMnBO3/C的交流阻抗谱图
Fig. 8 AC impedance spectra of LiMnBO3 and LiMnBO3/C
表2 等效电路拟合计算结果
Table 2 Parameters obtained from equivalent circuit fitting of experimental data
3 结论
1) 通过喷雾干燥法成功制备出了LiMnBO3和LiMnBO3/C样品,在0.025C倍率下LiMnBO3与LiMnBO3/C样品首次放电比容量分别为168.78和200.41 mA·h/g,PEG-6000作为碳源制备出的LiMnBO3/C样品电化学性能得到了明显的改善,并且LiMnBO3/C样品在0.025C、0.2C和1C倍率下循环50次后容量仍然为160.88、118.78和92.7 mA·h/g,显示出较好的循环性能。
2) PEG-6000不仅可作为碳源,而且在烧结过程中限制了颗粒的长大,分解的碳均匀地包覆在颗粒表面,形成了导电良好的碳网,提高了材料的电子电导率和离子电导率,从而提高了样品的电化学性能。
REFERENCES
[1] LEGAGNYER V, AN Y, MOSBAH A, PORTAL R, SALLEAllE A L L, VERBAERE A, GUYOMARD D, PIFFARD Y. LiMBO3 (M=Mn, Fe, Co): Synthesis, crystal structure and lithium deinsertion/insertion properties[J]. Solid State Ionics, 2001, 139: 37-46.
[2] TAO L, NEILSON J R, MELOT B C, MCQUEEN T M, MASQUELIER C, ROUSSE G. Magnetic structures of LiMBO3 (M=Mn, Fe, Co) lithiated transition metal borates[J]. Inorganic Chemistry, 2013, 52(20): 11966-11974.
[3] LIN Zhi-ping, ZHAO Yu-jun, ZHAO Yan-ming. First-principles study of the structural, magnetic, and electronic properties of LiMBO3 (M=Mn, Fe, Co)[J]. Physics Letters A, 2012, 376(3): 179-184.
[4] 唐安平, 钟倩雯, 胡拥军, 刘立华, 徐国荣. 锂离子电池正极材料LiMBO3研究进展[J]. 电源技术, 2015, 39(7): 1533-1579.
TANG An-ping, ZHONG Qian-wen, HU Yong-jun, LIU Li-hua, XU Guo-rong. Research progress in LiMBO3 (M=Mn, Fe, Co) cathode materials for Li ion batteries[J]. Chinese Journal of Power Sources, 2015, 39(7): 1533-1579.
[5] 张艳慧, 粟 智, 阿孜古丽·木尔赛力木, 翁之望. 锂离子电池正极材料LiMnBO3的研究现状[J]. 化工新型材料, 2012, 40(7): 7-9.
ZHANG Yan-hui, SU Zhi, ARZURUL M, WENG Zhi-wang. Research progress of LiMBO3 as anode material for lithium ion battery[J]. New Chemical Materials, 2012, 40(7): 7-9.
[6] MUSLIM A, MA Ting, SU Zhi, NIJAT I. Structural feature and electrochemical performance of h-LiMnBO3 and its carbon coated material prepared by microwave synthesis[J]. Rare Metal Materials and Engineering, 2014, 43(9): 2095-2099.
[7] TANG An-ping, HE Dong-hua, HE Ze-qiang, XU Guo-rong, SONG Hai-shen, PENG Rong-hua. Electrochemical performance of LiMnBO3/C composite synthesized by a combination of impregnation and precipitation followed by annealing[J]. Journal of Power Sources, 2015, 275(275): 888-892.
[8] MA Rui, SHAO Lian-yi, WU Kai-qiang, LAO Meng-meng, SHUI Miao, CHEN Cheng, WANG Dong-jie, LONG Neng-bing, REN Yuan-long, SHU Jie. Electrochemical behaviors of hexagonal LiMnBO3 as lithium storage host material for lithium-ion batteries[J]. Ceramics International, 2013, 39(8): 9309-9317.
[9] KIM J C, MOORE C J, KANG B, HAUTIER G, JAIN A, CEDER G. Synthesis and electrochemical properties of monoclinic LiMnBO3 as a Li intercalation material[J]. Journal of the Electrochemical Society, 2011, 158(3): A309-A315.
[10] ZHAO Li-wei, LI R K. Study on a multifunctional crystal LiMnBO3[J]. Materials Research Bulletin, 2013, 48(2): 277-280.
[11] CHEN Ling, ZHAO Yan-ming, AN Xiao-ning, LIU Jian-min, DONG You-zhong, CHEN Ying-hua, KUANG Quan. Structure and electrochemical properties of LiMnBO3 as a new cathode material for lithium-ion batteries[J]. Journal of Alloys and Compounds, 2010, 494(1/2): 415-419.
[12] LEE K J, KANG L S, UHM S H, YOON J S, KIM D W, HONG H S. Synthesis and characterization of LiMnBO3 cathode material for lithium ion batteries[J]. Current Applied Physics, 2013, 13(7): 1440-1443.
[13] LEE Y S, LEE H K. Structure and electrochemical behavior of LiMnBO3 synthesized at various temperatures[J]. Electronic Materials Letters, 2014, 10(1): 253-258.
[14] 李 琳, 郑 浩, 程劲松, 冯传启, 王石泉. LiMnBO3/C正极材料的合成及电化学性能的研究[J]. 电源技术, 2015, 39(2): 249-334.
LI Lin, ZHENG Hao, CHENG Jin-song, FENG Chuan-qi, WANG Shi-quan. Synthesis and electrochemical performance of cathode material LiMnBO3/C[J]. Chinese Journal of Power Sources, 2015, 39(2): 249-334.
[15] ZHONG Sheng-kui, WANG You, LIU Jie-qun, WANG Jian. Synthesis of LiMnPO4/C composite material for lithium ion batteries by sol-gel method[J]. Transactions of Nonferrous Metals Society of China, 2012, 22(10): 2535-2540.
[16] AFYON S, KUNDU D, KRUMEICH F, NESPER R. Nano LiMnBO3—A high-capacity cathode material for Li-ion batteries[J]. Journal of Power Sources, 2013, 224(4): 145-151.
[17] KARTHIKEYAN K, LEE Y S. Microwave synthesis of high rate nanostructured LiMnBO3 with excellent cyclic behavior for lithium ion batteries[J]. RSC Advances, 2014, 60(4): 31851-31854.
[18] 唐安平, 刘立华, 徐国荣, 申 洁, 令玉林. 锂离子电池硼酸盐电极材料的研究进展[J]. 应用化学, 2012, 29(11): 1221-1230.
TANG An-ping, LIU Li-hua, XU Guo-rong, SHEN Jie, LING Yu-ling. Progress in borate electrode materials for lithium ion batteries[J]. Chinese Journal of Applied Chemistry, 2012, 29(11): 1221-1230.
[19] LI Shou-li, XU Li-qiang, LI Guang-da, WANG Meng, ZHAI Yan-jun. In-situ controllable synthesis and performance investigation of carbon-coated monoclinic and hexagonal LiMnBO3 composites as cathode materials in lithium-ion batteries[J]. Journal of Power Sources, 2013, 236(25): 54-60.
[20] LEE Y S, LEE H K. Improved lithium storage capacities of LiMnBO3/C via simple high-energy milling[J]. Materials Letters, 2014, 132(10): 401-404.
[21] STAFEEVAZ V S, DROZHZHIN O A, PANIN R V, FILIMONOV D S, FABRICHNYI P B, YASHINA L V, KHASANOVA N R, ANTIPOV E V. The effect of LiFeBO3/C composite synthetic conditions on the quality of the cathodic material for lithium-ion batteries[J]. Russian Journal of Electrochemistry, 2015, 51(7): 619-626.
[22] WANG Ren-heng, LI Xin-hai, WANG Zhi-xing, GUO Hua-jun, HUANG Bin. PEG-combined liquid phase synthesis and electrochemical properties of carbon-coated Li3V2(PO4)3[J]. Transactions of Nonferrous Metals Society of China, 2015, 25(4): 1241-1247.
[23] ZHONG Sheng-kui, CHEN Wei, WU Ling, LIU Jie-qun. A PEG-assisted rheological phase reaction synthesis of 5LiFePO4·Li3V2(PO4)3/C as cathode material for lithium ion cells[J]. Ionics, 2012, 18(5): 523-527.
[24] 曹 亮, 王安安, 艾立华, 贾 明, 刘业翔. 石墨烯在锂离子电池材料性能优化中的应用[J]. 中国有色金属学报, 2016, 26(4): 807-820.
CAO Liang, WANG An-an, AI Li-hua, JIA Ming, LIU Ye-xiang. Application of grapheme in performance optimization of lithium ion battery materials[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(4): 807-820.
[25] 冯福山, 方海升, 杨 斌, 马文会, 戴永年. 升温速率对高电压正极材料LiNi0.5Mn1.5O4晶粒形貌及电化学性能的影响[J]. 中国有色金属学报, 2016, 26(2): 347-353.
FENG Fu-shan, FANG Hai-sheng, YANG Bin, MA Wen-hui, DAI Yong-nan. Effect of heating rate on crystal morphology and electrochemical performance of high voltage cathode material LiNi0.5Mn1.5O4[J]. The Chinese Journal of Nonferrous Metals, 2016, 26(2): 347-353.
[26] 义丽玲, 王先友, 白艳松, 王 钢, 刘梅红. 煅烧温度对溶 胶-凝胶法制备的Li2FeSiO4/C正极材料性能的影响[J]. 中国有色金属学报, 2017, 27(2): 289-294.
YI Li-ling, WANG Xian-you, BAI Yan-Song, WANG Gang, LIU Mei-hong. Influences of sintering temperature on performances of Li2FeSiO4/C cathode material synthesized by sol-gel method[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(2): 289-294.
Synthesis and electrochemical properties of LiMnBO3/C as cathode material for Li-ion batteries
CHEN Wei1, ZHANG Hua3, ZHANG Xiao-ping2, LIU Jie-qun2, LIU Shi-jun1, ZHONG Sheng-kui2
(1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. School of Iron and Steel, Soochow University, Suzhou 215021, China;
3. Shanghai Shanshan Technology Co., Ltd., Shanghai 201209, China)
Abstract: LiMnBO3 and its carbon coated material LiMnBO3/C were synthesized via spray-drying method using LiOH·H2O, Mn(CH3COO)2·4H2O and H3BO3, as starting materials and polyethylene glycol 6000 (PEG-6000) as carbon resource. XRD results indicate that the samples have hexagonal crystal structure (h-LiMnBO3). The electrochemical tests show that the LiMnBO3/C particles delivers a high first discharge capacity of 135.21 mA·h/g at 0.5C rate within 1.0-4.80 V and retaining a discharge capacity of 109.69 mA·h/g after 50 cycles, while the LiMnBO3 composite displays a first discharge capacity of 63.28 mA·h/g and a capacity remaining of 31.15 mA·h/g after 50 cycles. The carbon-coated LiMnBO3 could effectively improve the electrochemical performance.
Key words: cathode material; LiMnBO3; spray-drying; carbon-coated
Foundation item: Projects(51574168, 51574170, 51404156) supported by the National Natural Science Foundation, China; Project(BK20141231) supported by the Natural Science Foundation of Jiangsu Province, China; Project(SYG201512) supported by the Science and Technology Plan Projects of Suzhou, China
Received date: 2017-01-03; Accepted date: 2017-05-23
Corresponding author: LIU Shi-jun; Tel: +86-731-88877364; E-mail: shijunliu@csu.edu.cn
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51574168,51574170,51404156);江苏省自然科学基金资助项目(BK20141231);苏州市科技计划项目(SYG201512)
收稿日期:2017-01-03;修订日期:2017-05-23
通信作者:刘士军,教授,博士;电话:0731-88877364,E-mail:shijunliu@csu.edu.cn
摘 要:以LiOH·H2O、Mn(CH3COO)2·4H2O和H3BO3为原料,聚乙二醇6000(PEG-6000)为碳源,采用喷雾干燥法合成LiMnBO3和LiMnBO3/C正极材料。XRD测试表明,两种样品均为单一的六方晶体结构LiMnBO3(h-LiMnBO3);电化学测试表明,在电压范围1.0~4.8 V内,LiMnBO3在0.5C倍率下的首次放电比容量为63.28 mA·h/g,而LiMnBO3/C的首次放电比容量高达135.21 mA·h/g;循环50次后,两者比容量分别为31.15 mA·h/g和109.69 mA·h/g。碳源的加入有效地提升了LiMnBO3的电化学性能。
[4] 唐安平, 钟倩雯, 胡拥军, 刘立华, 徐国荣. 锂离子电池正极材料LiMBO3研究进展[J]. 电源技术, 2015, 39(7): 1533-1579.
[5] 张艳慧, 粟 智, 阿孜古丽·木尔赛力木, 翁之望. 锂离子电池正极材料LiMnBO3的研究现状[J]. 化工新型材料, 2012, 40(7): 7-9.
[14] 李 琳, 郑 浩, 程劲松, 冯传启, 王石泉. LiMnBO3/C正极材料的合成及电化学性能的研究[J]. 电源技术, 2015, 39(2): 249-334.
[18] 唐安平, 刘立华, 徐国荣, 申 洁, 令玉林. 锂离子电池硼酸盐电极材料的研究进展[J]. 应用化学, 2012, 29(11): 1221-1230.
[24] 曹 亮, 王安安, 艾立华, 贾 明, 刘业翔. 石墨烯在锂离子电池材料性能优化中的应用[J]. 中国有色金属学报, 2016, 26(4): 807-820.


