网络首发时间: 2019-07-28 18:45
镍掺杂TiO2纳米管的制备及光催化性能研究
东北大学冶金学院
摘 要:
通过前驱体水热法成功制备出不同镍掺杂比例的二氧化钛纳米管粉体,利用场发射扫描电镜(FESEM)、X射线衍射仪(XRD)、X射线光电子能谱(XPS)、紫外可见吸收光谱(UV-Vis)等对其进行检测表征。制备出的镍掺杂二氧化钛纳米管具备相对分离和独立的管状形貌,管径约10 nm,其对应的EDS谱图中出现了镍的特征峰。经500℃热处理后样品呈锐钛矿和晶红石的混晶结构。XPS检测表明掺杂样品中镍主要以Ni2+形式存在。样品的紫外可见吸收光谱表明镍掺杂样品的光吸收发生红移且镍掺杂明显提升了样品的光吸收能力。光催化测试表明适宜的镍掺杂能够有效降低二氧化钛光生电子和空穴的复合率,从而提升了纳米管样品对亚甲基蓝的降解率。其中2%镍掺杂纳米管对亚甲基蓝显示出最高的2 h降解率,达到95.7%,而过量的镍掺杂则会对其催化性能产生抑制作用。
关键词:
中图分类号: O643.36;O644.1;TB383.1
作者简介:田昂(1982-),男,辽宁抚顺清原满族自治县人,博士,讲师,研究方向:纳米氧化物材料,E-mail:tiana@smm.neu.edu.cn;;*杨合,教授,电话:13002436218,E-mail:yangh@smm.neu.edu.cn;
收稿日期:2019-06-12
基金:广东省新能源和可再生能源研究开发与应用重点实验室开放基金项目(Y607s31001)资助;
Preparation and Photocatalytic Properties of Ni Doped TiO2 Nanotube
Tian Ang Shi Xiaoguo Tan Haocun Li Bingxuan Ma Jiawei Yang He
School of Metallurgy,Northeastern University
Abstract:
At present,element doping has been proved to be an effective means to solve the shortcomings of TiO2 photocatalytic materials such as narrow light response range and low quantum efficiency,and to optimize and improve the catalytic performance of TiO2.Element doping introduces various transition metal or non-metal electronic active bodies into the TiO2 crystal lattice to reduce the forbidden band width,thereby improving the photocatalytic efficiency of TiO2 and shifting the maximum excitation wavelength to the near ultraviolet or visible light region.Therefore,the research on suitable doping elements and the doping optimization mechanism have become the current research hotspot.Among all the transition metals,Ni element has become the focus of this article as a potential doping element due to its abundant reserves,environmental friendliness,and low price.Titanium dioxide nanotube powder with different Ni doping ratios were successfully prepared by the precursor hydrothermal method and characterized by field emission scanning electron microscope(FESEM),X-ray diffraction(XRD),X-ray photoelectron spectroscopy(XPS),ultraviolet and visible spectrophotometry(UV-Vis).The photocatalytic performance of the sample was characterized by catalytic degradation of methylene blue under ultraviolet light and simulated sunlight.FESEM and TEM results showed that the diameter of the undoped TiO2 nanotubes was about 10 nm,and the tubes were relatively independent and entangled with each other.The appropriate amount of nickel doping would not affect the structure and size of the nanotubes.Energy dispersive X-ray spectrum(EDX)analysis showed that Ni doping in TiO2 nanotubes was achieved during the preparation process,and the Ni content slightly lower than the doping ratio was mainly due to the loss of nickel during the preparation process.XRD analysis showed that nanotube samples were transformed into a mixed crystal structure of anatase and rutile after heat treatment.Since the proportion of Ni in the doped sample was low,the characteristic peak corresponding to Ni was not detected.The characteristic peaks of C 1 s,Ti 2 p,O 1 s,and Ni 2 p were detected in the XPS spectrum.The characteristic peaks of Ni 2 p at 855.37 and 872.87 eV were corresponding to the Ni 2 p3/2 and Ni 2 p1/2 peaks of Ni2+ in NiO,respectively,which indicated that the nickel element mainly existed in the form of NiO.The characteristic peaks at 861.45 and 879.51 eV corresponded to the Ni 2p3/2 and Ni 2p1/2 peaks of Ni3+ with a small amount of Ni2O3 produced during the preparation process.The ultraviolet-visible absorption spectrum showed that as the nickel doping ratio increases,the absorption intensity of nanotube powder sample in the visible light region gradually increased,and the sample with 4% nickel doping reached the strongest.The absorption of the sample had a significant red shift with the increase of the doping ratio,which was mainly because Ni doping into the TiO2 lattice,generating a new energy level on the TiO2 valence band,thereby reducing the band gap of the sample.Therefore,nickel doping was beneficial to improve the absorption performance of the TiO2 nanotube sample in the visible light region.Experiments on the catalytic degradation of methylene blue by different nanotube samples under simulated sunlight and ultraviolet light showed that the catalytic efficiency of the samples under ultraviolet light was significantly higher than that under simulated sunlight.Under simulated sunlight and ultraviolet light,the degradation rate of each sample to methylene blue gradually increased with the increase of the Ni doping ratio.However,when the Ni doping amount reached 4%,the catalytic efficiency decreased to a certain extent.The possible mechanism of the modification effect of nickel doping in TiO2 nanotubes was as follows:Since the conduction band position of NiO(2.1 eV)was lower than that of TiO2(3.05 eV),the conduction band electrons on TiO2 would transition to the conduction band of NiO which has lower energy.This reduced the recombination rate of electrons and holes in TiO2,and ultimately improved the catalytic performance of TO2.However,when the nickel doping ratio was further increased,the catalytic performance of the composite sample would decrease.The main reasons for this phenomenon included:On the one hand,when the Ni doping ratio of the system increased,the relative content of TiO2 would become smaller.The catalytic effect of the system was weakened;secondly,excessive nickel doping would become the recombination center of electrons and holes to increase the probability of photogenerated electron-hole recombination,thereby inhibiting the catalytic effect of the system.The nickel-doped titanium dioxide nanotube powder was prepared by the precursor hydrothermal method.The diameter of the nanotube was about 10 nm,and it had a mixed crystal structure of anatase and rutile after heat treatment.Nickel doping enhanced the light absorption capacity of TiO2 nanotubes and made its light absorption redshift.A proper amount of nickel doping was beneficial to inhibit the recombination of photo-generated electrons and holes,thereby enhancing the catalytic activity of the material.The 2% Ni doped nanotube showed the highest 2 h degradation rate for methylene blue,which was 95.7%,while excessive Ni doping would have inhibitory effect on its catalytic performance.
Keyword:
titanium dioxide nanotubes; Ni doping; hydrothermal method; photocatalysis;
Received: 2019-06-12
Ti O2纳米管是纳米Ti O2的一种特殊结构,具有更大的比表面积和独特的管状结构,表现出更好的光催化活性
当前,元素掺杂已被证实是一种优化提升Ti O2催化性能的有效手段。掺杂过程通过将各种过渡金属或非金属的电子活性体引入Ti O2的晶格,使其禁带宽度降低,从而提高Ti O2的光催化效率以及使其最大激发波长转移到近紫外或者可见光区
在众多过渡金属中,Ni元素由于其地壳储量丰富,环境友好性,较低的价格等优势作为极具潜力的掺杂元素引起了人们的注意。本文以Ni Cl2为镍盐,采用前驱体水热法制备出镍掺杂二氧化钛纳米管粉体。通过扫描电镜(SEM)、X射线衍射(XRD)、X射线光电子能谱(XPS)、紫外可见分光光度计(UV-Vis)等手段对其进行表征并对其光催化性能进行了研究。
1 实验
1.1 镍掺杂二氧化钛纳米管的制备
镍掺杂Ti O2纳米管粉体通过溶胶凝胶法制备得到:将25 ml钛酸四丁酯溶解于100 ml乙醇中并磁力搅拌30 min。将5 ml浓硝酸(质量分数为68%),25 ml无水乙醇、25 ml去离子水和不同质量比例的氯化镍(相比于前驱体Ti O2,镍掺杂质量比分别为0%,1%,2%,4%)充分搅拌混合。将两种溶液混合搅拌,陈化6 h得到淡黄色透明凝胶。将所得凝胶真空干燥研磨得到前驱体。称取2 g前驱体,与90 ml、10 mol·L-1的Na OH溶液混合,磁力搅拌24 h。然后将该悬浊液转入高温高压反应釜中(填充度90%左右),以120°C保温24 h。随后将样品真空抽滤分离,将滤渣在0.1 mol·L-1的稀硝酸溶液中酸洗12 h后真空抽滤分离,用去离子水洗至中性并真空干燥。最后将样品在马弗炉中以500°C退火3 h,得到镍掺杂Ti O2纳米管粉体。
1.2 样品的表征和光催化性能
以未掺杂Ti O2纳米管粉体做为对照组材料,各样品的表面形貌和成分通过场发射扫描电镜(Zeiss Ultra,Zeiss)和配套的能谱仪检测表征;样品的晶体结构和化学成分通过X射线衍射(XRD,D8211,Huber)和X射线光电子能谱(XPS,Escalab250,Thermo VG)检测表征。样品的光吸收性能和催化性能通过紫外可见分光光度计(UV-Vis,UV2550,Shimadzu)测量。样品的光催化性能通过催化降解亚甲基蓝来表征。配置30 mg·L-1的亚甲基蓝溶液。分别取50 mg纳米管样品置于100 ml亚甲基蓝溶液中,避光搅拌60 min达到吸附—解吸平衡。随后分别施加紫外光和模拟太阳光照并开始计时,测量不同光照下各纳米管样品对亚甲基蓝的降解率。
2 结果与讨论
图1和表1为不同Ni掺杂比例Ti O2纳米管的形貌图以及EDS元素分析表,本文中未掺杂、1%,2%,4%镍掺杂的Ti O2纳米管样品分别简写为TNT,1%Ni-TNT,2%Ni-TNT,4%Ni-TNT。由图1可知,未掺杂Ti O2纳米管管径约为10 nm,管与管间相对独立且彼此纠缠交错。而镍掺杂之后的Ti O2纳米管管长、管径并无明显变化,由此说明适量的镍掺杂不会影响纳米管的结构形貌。由图1(e)中样品的透射电镜可知,制备出的纳米管边缘颜色较深,中部颜色较浅,呈现出纳米管形貌。由表1可知,各掺杂样品中均检测出Ni元素,由此说明制备过程实现了Ni掺杂,与此同时,Ni含量略低于掺杂比例,这主要是由于制备过程中的镍损失造成的。
图2为不同样品的XRD谱图。各样品的特征峰较为尖锐,表明各纳米管样品经热处理后转变为晶体结构,各样品中出现锐钛矿和晶红石的特征峰。由于掺杂样品中Ni的比例较低,因此未在样品中检测到Ni对应的特征峰。
为了对制备样品的元素种类,化学价态等信息进行确定,我们对样品进行了XPS测试,结果如图3所示。由XPS全谱图3(a)可知,谱图中主要出现C 1s,Ti 2p,O 1s,Ni 2p的特征峰。其中C元素主要来自表面吸附的二氧化碳或者含碳化合物杂质,通常用于XPS的能量校正。用Shirly曲线扣除背底后利用高斯函数对样品的Ti 2p,O 1s,Ni 2p高分辨谱峰进行拟合处理。图3(b)中,位于458.31和464.02 e V的结合能峰,对应着Ti O2中Ti4+的2p3/2峰和2p1/2峰
图1 纳米管样品的TEM照片
Fig.1 TEM images of prepared nanotube samples
(a)Ti O2nanotube;(b)1%Ni-Ti O2nanotube;(c)2%Ni-Ti O2nanotube;(d)4%Ni-Ti O2nanotube;(e)TEM image of 2%NiTi O2nanotube
表1 纳米管样品EDS分析汇总表 下载原图
Table 1 Summary table of EDS analysis of prepared nanotube samples

图2 纳米管样品的XRD谱图
Fig.2 XRD patterns of prepared nanotube samples
图4为不同Ni掺杂比例的样品的紫外可见吸收图谱。随着镍掺杂比例的升高,各纳米管粉体样品在可见光区域的吸收强度逐渐增加,4%镍掺杂比例的样品达到最强。与此同时,样品的吸收随着掺杂比例的提升发生明显红移,这主要是因为Ni掺杂进入Ti O2晶格,在Ti O2价带上产生新的能级,由此减小了样品的禁带宽度
图5为不同纳米管样品在模拟太阳光和紫外光下对亚甲基蓝的降解曲线和降解动力学拟合曲线。紫外光照下样品的催化效率明显高于模拟太阳光下。在模拟太阳光和紫外光下,各样品对亚甲基蓝的降解率随着Ni掺杂比例的提升逐步提升,然而当镍掺杂量达到4%时,催化效率反而出现了一定程度的下降。由图5(c,d)可知,各样品对亚甲基蓝的拟合降解动力学曲线符合伪一级动力学方程,方程如下:
式中,C0为Methylene blue(MB,亚甲蓝)初始浓度,C为光照t时间后的MB浓度,t为光照时间,k为反应速率常数。由各样品对应的反应速率常数可知,各样品的催化速率随着掺杂比例的提升而提升,但当掺杂比例过高时,催化速率反而下降。综上所述,2%-Ni掺杂比例样品显示出最高的催化性能。
图3 Ni-Ti O2纳米管样品的XPS谱图
Fig.3 XPS spectra of Ni-Ti O2nanotube
(a)Full-scan;(b)Ti 2p;(c)O 1s;(d)Ni 2p
图4 纳米管样品的UV-Vis吸收谱图
Fig.4 UV-Vis absorption patterns of prepared nanotube samples
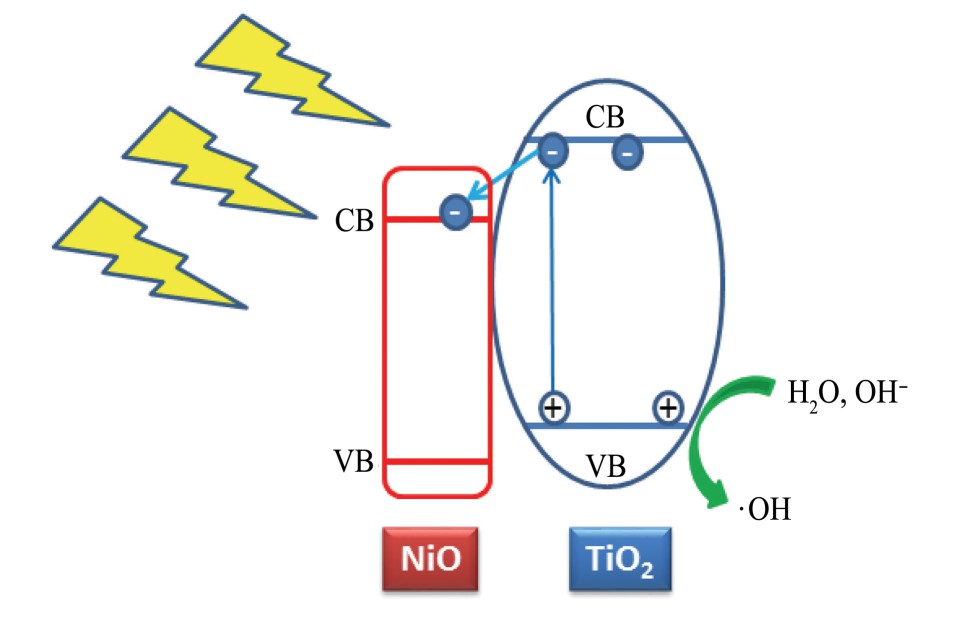
图6为镍掺杂Ti O2纳米管的催化原理示意图。由图6可见,镍掺杂对Ti O2纳米管的修饰作用的可能机制如下:由于Ni O的导带位置(2.1 e V)低于Ti O2的导带位置(3.05 e V)
3 结论
通过前驱体水热法制备出镍掺杂二氧化钛纳米管粉体,纳米管管径约为10 nm,热处理后样品为锐钛矿和晶红石的混晶结构。镍掺杂增强了纳米管的光吸收能力并使其发生红移。样品的催化实验表明适量的镍掺杂有利于抑制光生电子和空穴的复合,从而增强了材料的催化活性。而过量的镍掺杂则会抑制材料的催化性能。
图5 纳米管样品对亚甲基蓝的降解曲线
Fig.5 Degradation efficiency of prepared nanotube samples for MB
(a)Simulated sunlight;(b)UV light and degradation kinetics plots;(c)Simulated sunlight,(d)UV light
图6 Ni掺杂Ti O2纳米管的催化原理示意图
Fig.6 Catalysis mechanism schematic diagram of the Ni-Ti O2nanotube
参考文献