Localized corrosion of copper alloys in China seawater for 16 years
ZHAO Yue-hong(赵月红), LIN Le-yun(林乐耘), CUI Da-wei(崔大为)
(General Research Institute for Nonferrous Metals, Beijing 100088, China)
Abstract:
The regulation of localized corrosion of 2 kinds of copper and 17 kinds of copper alloys exposed in seawater of Qingdao, Zhoushan, Yulin and Xiamen for 16 years has been studied. Results show that during immersion copper alloys suffer from pitting corrosion due to high temperature and marine living adhesion at Yulin, and to the higher velocity of seawater containing sand at Zhoushan. However, the seawater of Xiamen inhibits the pitting corrosion of copper alloys. No pitting corrosion is observed on copper alloy plates tested there. The copper alloys suffer from more serious pitting corrosion in the tide zone than that in the immersion zone at Qingdao after long time exposure.
Key words:
copper alloy; seawater exposure; pitting corrosion CLC number: TG146;
Document code: A
1 INTRODUCTION
Copper and copper alloys have excellent anti-corrosion and antifouling properties. Thus they were applied in marine engineering and sea exploration very widely[1-3]. The corrosion date of copper and its alloy in marine have been reported in many documents[4-7]. These reports showed that the corrosion of copper and its alloys in different seawater were not the same due to the different characteristics of the seawater. Current research works about anticorrosion and antifouling mechanism of copper and copper alloys focused on the corrosion product film[8-15]. In this paper, the corrosion behaviors of 2 kinds of copper and 17 kinds of copper alloys exposed in seawater of Qingdao, Zhoushan, Yulin and Xiamen for 16 years have been studied.
2 EXPERIMENTAL
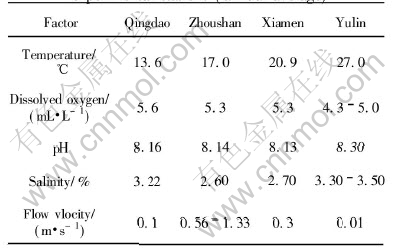
In this work, samples were exposed in seawater following the instruction of ISO11306. The exposure periods were one year, two years, four years, eight years and sixteen years. Test zones covered immersion zone, tide zone and splash zone. The properties of seawater in experimental stations (annual average) are shown in Table 1.
In this experiment, 12 kinds of plates and 7 kinds of tubes of copper and copper alloy were studied. Chemical composition and mechanical property of samples are shown in Table 2. The plates were cut to 200mm×100mm.The longer
Table 1 Properties of seawater in experimental stations (annual average)
side was perpendicular to the rolling direction of plates. Tubes were cut to 200mm long. Samples were degreased before exposure. The process consists of washing with gasoline, washing with metal detergent, rinsing-dewatering with anhydrous alcohol, drying and packaging. And then the original defect on the surface of samples were checked and recorded and the samples were measured precisely before the experiment. Samples were treated following ISO11306 after the test period.
3 RESULTS AND DISCUSSION
3.1 Corrosion regulation of copper and copper alloy in immersion zone
As shown in Fig.1 and Fig.2, the average corrosion rate of copper alloy plates declined in sequence of Zhoushan, Yulin and Xiamen, Qing dao. And the average pit depth of copper alloy plates decreased progressively in the order of Yulin, Zhoushan, Qingdao and Xiamen.
Table 2 Chemical composition and mechanical properties of copper alloys
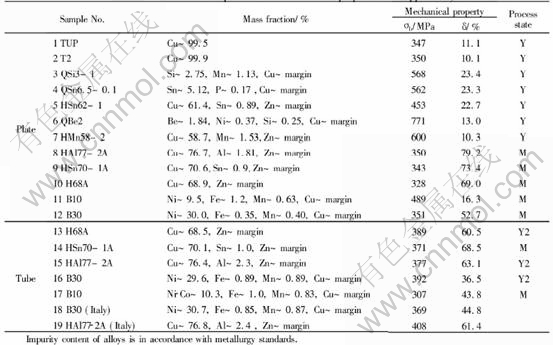
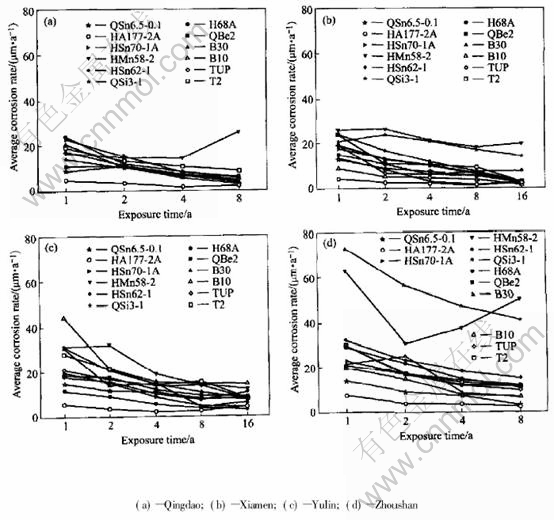
Fig.1 Average corrosion rate of copper alloy plates exposed in immersion zone of seawater (calculated from loss)
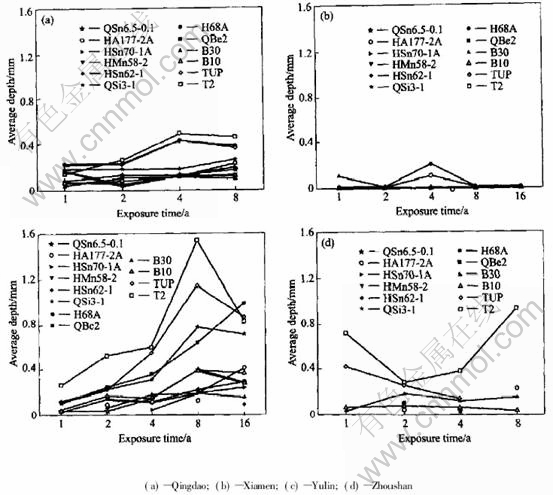
Fig.2 Average depth of 10 deepest pits on copper alloy plates exposed in immersing zone of seawater
The average corrosion rate of copper alloy plates, such as QSi3-1, HAl77-2A, HMn58-2, HSn62-1, H68A, was the largest in Zhoushan,but the average pit depth in Zhoushan (0.05-0.94mm)was lower than that in Yulin (0.03-1.54mm), which illustrated that the corrosion of copper alloys in the seawater of Zhoushan was mainly general corrosion in comparison with that in Yulin. In Zhoushan, seawater contained lots of sand, and the velocity was largest in all the stations. The pit depth of copper alloy plates was relatively lower due to erosion corrosion of seawater containing sand. QSi3-1, HMn58-2 are more sensitive to erosion corrosion of sand-containing seawater in all the samples. The corrosion rates of QSn6.5-0.1 and HAl77-2A in Zhoushan were a bit different from those in other stations. This indicates that QSn6.5-0.1 and HAl77-2A are more optimal in resistance to erosion corrosion than the others.
Compared with other stations, the temperature of Yulin seawater is much higher. The corrosion behavior of some copper alloys is sensitive to temperature, such as copper, cupronickel alloy and tin bronze. The average corrosion rate in the immersion zone of Yulin is the highest. Moreover, the average pit depth of these alloys is the deepest in Yulin. So, these alloys have serious pit corrosion because of high temperature. In addition, because it is warm in Yulin, the marine creature grows very vigorously. The marine creature adhesion also contributes to localized corrosion.
The seawater in Xiamen is very special. As shown in Fig.1, the average corrosion rate of copper alloy plates is not the lowest in Xiamen, and even the corrosion rate of some plates in Xiamen is higher than that of in Yulin, for example, H68A,QSi3-1. But all copper alloy plates in Xiamen seawater have not suffered from local corrosion after being exposed for one year, two years, four years, eight years and even sixteen years, except some copper alloy plates with a few small and shallow pits after 4 years exposure.
And it can be inferred that the local corrosion behavior of Xiamen seawater on copper alloy plates is different from those in other stations because of the different chemical compositions and the current velocities. Because Jiulong River flows into Xiamen sea, the salinity of Xiamen seawater is only 2.70%, lower than that of Yulin seawater and Qingdao seawater(Table 3). Moreover the average tide of half day in Xiamen is 3.9m, higher than that in Zhoushan, where the average tide of half day is 2.08m, and Yulin, 1.6m. Thus, the seawater velocity is higher in Xiamen when the tide is flowing or ebbing. The high speed seawater can result in corrosion of copper alloy. However, the less salinity inhibits the localized corrosion of copper alloy plates. Detail reason needs further research.
However, the inhibition is limited. As shown in Table 4, the average depth of pits on Cu70Ni30 tubes is 0.32mm after being exposed for two years, and 0.23mm for four years, and the depth of the deepest pit is 0.44mm for two years, and 0.41mm for four years in the immersion zone of Xiamen seawater. The other copper alloy tubes also have measurable pits after exposed in Xiamen seawater. That shows Xiamen seawater has less or no inhibition on copper alloy tubes. The processing of the plates is different from that of the same kind of tubes. So the microstructure is different and the surface condition is not the same. And then the corrosion properties of the plates and the tubes are different. The result indicates that the plates are more anticorrosive than the tubes. With the same kind of copper alloys, especially in the first several years, the average corrosion rate of the tubes is obviously higher than that of the plates. Inhibition on local corrosion of copper alloy in Xiamen seawater is very limited, and would be weakened when the sensitivity of alloy to local corrosion is strengthened.
Temperature in Qingdao seawater is lower compared with that in other seawaters. And it results in the lower average corrosion rate of copper alloy plate in Qingdao than in the other station. Except HMn58-2 brass, the average corrosion rates of copper alloys decrease with time. Except HMn58-2 and HAl77-2A brass, the data of average corrosion rate of copper alloy plates are relatively more dispersive after exposed for one year, than for two years, four years and eight years. The average corrosion speed is 4.8-24μm/a after exposed for one year, 9.9-16μm/a after exposed for 2 years, 5.6-11μm/a after exposed for 4 years, 3.5-8.6μm/a after exposed for 8 years. All the average deepest pits depth are low (rang from 0.04mm to 0.46mm). Then it could be inferred that the localized corrosion of copper alloy plate is very light in Qingdao.
3.2 Corrosion behavior of copper alloy in different zones of test stations
In general, the corrosion of metal material is most serious in immersion zone, a little lighter in tide zone, and the least in splash zone. The corrosion of copper alloy also follows the same rule. The average corrosion rate and corrosion depth versus exposure time of copper alloy plate in the tide zone and splash zone are shown in Fig.3 to Fig.6. In tide zone, the average corrosion rate decreases in the order of Qingdao, Xiamen, Zhou shan, Yulin since the temperature increases in the same order. The volatilization of the water film on
Table 3 Corrosion data of some copper alloy plates exposed in immersion zone of seawater for 16 years
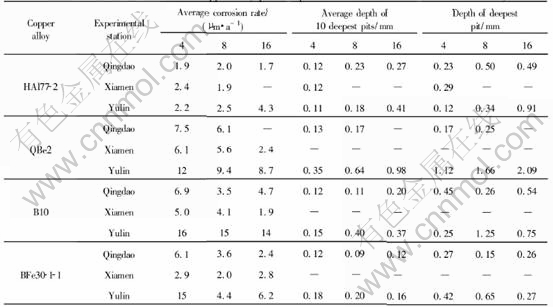
Table 4 Corrosion data of some copper alloy tubes exposed in immersion zone of seawater for 16 years
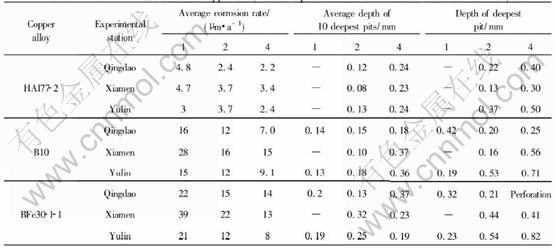
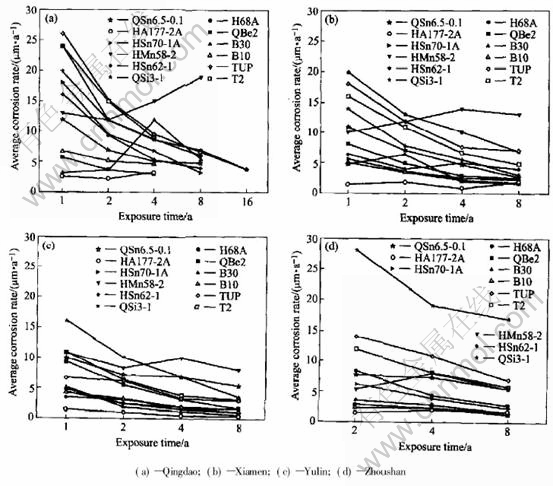
Fig.3 Average corrosion rate of copper alloy plates exposesed in mean tide zone of seawater (calculated from loss)
copper alloy plate surface in tide zone goes faster one by one. It is the time of alloy in the solution that decreases in the same order. Thus the corrosion rate decreases one by one. In the tide zone copper alloy plates have slight pit corrosion only at Qingdao and Yulin, and no local corrosion at Xiamen and Zhoushan. This depends on the characteristics of the seawater.
In splash zone, the average corrosion rate decreases by the order of Qingdao, Yulin, Xiamen, Zhoushan. Copper alloy has no local or pitting corrosion in the test station except Qingdao. The sur-
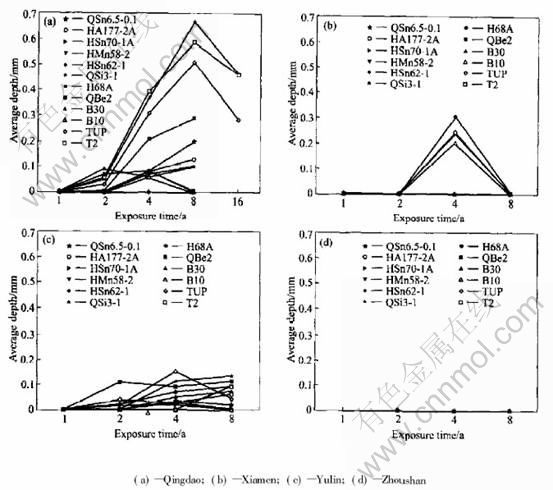
Fig.4 Average depth of 10 deepest pits on copper alloy plates exposed in mean tide zone of seawater
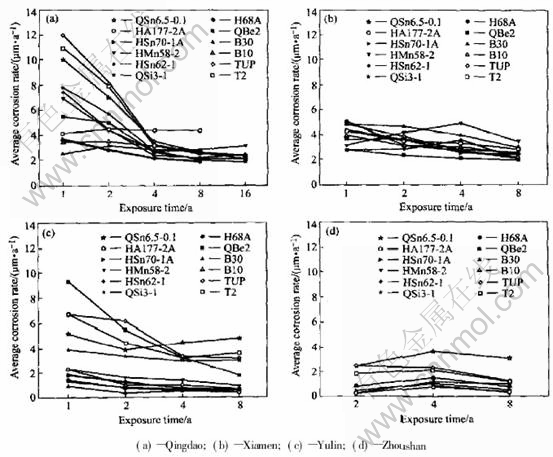
Fig.5 Average corrosion rate of copper alloy plates exposed in splashing range of seawater (calculated from loss)
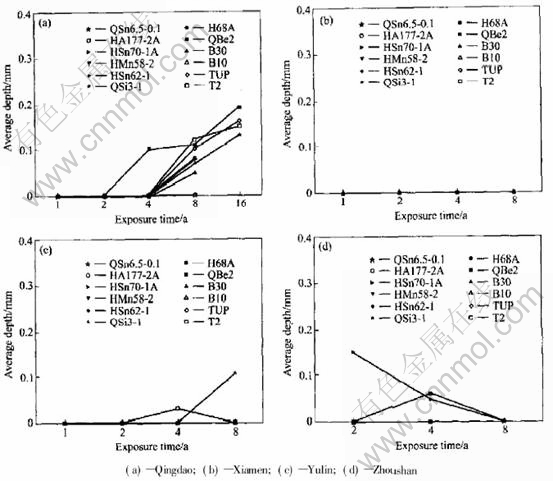
Fig.6 Average depth of 10 deepest pits on copper alloy plates exposed in splashing zone of seawater
face of copper alloy splashes by water more often than in the splash zone of Qingdao which is on the rock while Yulin, Xiamen and Zhoushan are on the flat place. So in the splash zone of Qingdao, the average corrosion rate is higher than that in other test stations, and has slight local corrosion, and the average pit depth is 0.05-0.2mm.
In the splash zone of Yulin, the average corrosion rate of copper and bronze was obviously higher than those of brass and cupronickel because copper and bronze were more sensitive to temperature and oxygen than brass and cupronickel.
The average corrosion rate and pit depth of copper alloy in the three test zones of Qingdao are shown in Fig.7 and Fig.8. It is obvious that corrosion behavior of copper alloy in Qingdao seawater is different from that in other test stations. In other test stations, generally, the average corrosion rate of copper alloy decreases in sequence of the immersion zone, the tide zone and the splash zone. But in Qingdao, the average corrosion rate has little difference between in the immersion zone and in the tide zone. In the immersion zone, the living creature adhesion is relatively little and it does not increase corrosion much for the low temperature. And the corrosion of copper alloy in the immersion zone is comparatively weakened. In the tide zone, the period in which alloy has the water film is longer because temperature is lower, and oxygen content is higher than in the immersion zone, so the corrosion of copper alloy in the tide zone is more serious. Therefore, the average corrosion rate in the tide zone shows no great difference from that in the immersion zone. However the corrosion rates in both of the two zones are more serious than that in the splash zone. The regulation of the average pit depth is basically the same. Just in the short time exposure (one year and two years), the average pit depth of copper alloy is smaller in the tide zone than that in the immersion zone. And in the later exposure (four years and eight years), the average pit depth is higher in the tide zone than in the immersion zone, especially for QSi3-1 and QSn6.5-0.1.
4 CONCLUSIONS
1) In the immersion zone, copper and copper alloys suffer from pitting corrosion due to high temperature and marine creature adhesion at Yulin, because of higher velocity of seawater containing sand at Zhoushan. And the seawater of
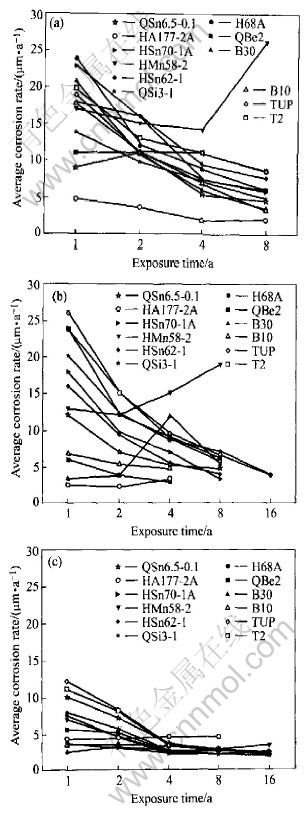
Fig.7 Average corrosion rate of copper alloy plates exposed in seawater of Qingdao
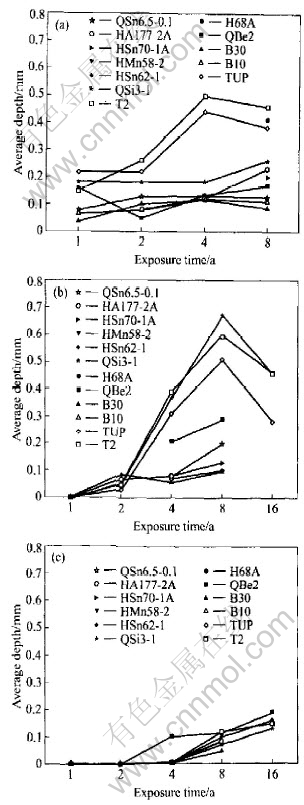
Fig.8 Average depth of 10 deepest pits on copper alloy plates exposed in seawater of Qingdao
Xiamen inhibits the pitting corrosion of copper alloys, so that no pitting corrosion is observed in copper alloy plates tested there. However, the inhibition is limited. The copper alloy tubes still suffer from pitting corrosion in the immersion zone of Xiamen seawater.
2) In the tide zone, the average corrosion rate of copper alloys decreases in sequence of Qingdao, Xiamen, Zhoushan, Yulin. And copper and copper alloy have pit corrosion only at Qingdao and Yulin.
3) The copper alloys suffer from more serious pitting corrosion in the tide zone than that in the immersion zone at Qingdao during the long exposure stage.
4) In the splash zone, the average corrosion rate of copper alloys decreases in sequence of Qingdao, Yulin, Xiamen, Zhoushan, and copper and copper alloy have pitting corrosion only at Qingdao.
ACKNOWLEDGEMENT
The authors greatly acknowledge the assistance of the staff in the test stations of Qingdao, Yulin, Xiamen, and zhoushan.
REFERENCES
[1]Schumacher M. Seawater Corrosion Handbook[M]. New Jersey: Park Ridge, 1979.98-104.
[2]Glover T G. Copper-nickel alloy for the construction of ship and boat hulls[J]. British Corrosion Journal, 1982, 17(4): 155-158.
[3]Agarwal D C. Effect of ammoniacal sea water on material properties of copper-nickel alloy[J]. British Corrosion Journal, 2002, 37(2): 105-110.
[4]Ijsseling F P, Prolenga L J P, Koster B H. The influence of alloy composition and microstructure on the corrosion behavior of copper-nickel alloys in seawater[J]. Werk Korro, 1983, 34: 167-178.
[5]Prolenga L J P, Ijsseling F P, Koster B H. Influence of temperature on corrosion product film formation in Cu-Ni10Fe in the low temperature range, I-corrosion rate as a function of temperature in well aerated seawater[J]. British Corrosion Journal, 1982, 17(4): 162-167.
[6]LIN Le-yun, XU Jie, ZHAO Yue-hong. Study on corrosion behavior of B10 alloy exposed to natural seawater[J]. Journal of Chinese Society for Corrosion and Protection, 2000, 20 (6): 361-367. (in Chinese)
[7]LIN Le-yun, ZHAO Yue-hong. Study on grain boundary structure and corrosion behavior of copper alloys[J]. Rare Metals, 2000, 19(4), 301-305.
[8]Schrader M E. Auger electron spectroscopic study of mechanism of sulfide-accelerated corrosion of Cu-Ni alloy in sea water[J]. Appli Surf Sci, 1982, (10): 431-441.
[9]ZHU Xiao-long, LIN Le-yun, LEI Ting-quan. Corrosion resistance of deformed Cu-Ni alloy in seawater [J]. Rare Metals, 1997, 16(1): 16-19. (in Chinese)
[10]ZHU Xiao-long, LEI Ting-quan. Characteristics and formation of corrosion product films of 70Cu-30Ni alloy in seawater[J]. Corrosion Science, 2002, 44: 67-79.
[11]LIU Shao-feng, LIN Le-yun. Transforming behavior of surface film of Cu-Ni alloy exposed to seawater[J]. Chinese Journal of Materials Research, 1998, 12(1): 20-24. (in Chinese)
[12]LIN Le-yun, WANG Xiao-hua, ZHAO Yue-hong. Influence of microstructure and surface condition on antifouling property of 90Cu-10Ni alloy in seawater[J]. Trans Nonferrous Met Soc China, 2001, 11(4): 563-566.
[13]LIN Le-yun, LIU Shao-feng, LIU Zeng-cai, et al. Surface and interface characteristics of Cu-Ni alloy corrosion in seawater[J]. Corrosion Science and Protection Technology, 1999, 11(1): 37-43. (in Chinese)
[14]LIN Le-yun, LIU Zeng-cai, ZHAO Yue-hong, et al. Study on marine corrosion and antifouling behavior of copper alloys exposed to sea areas in China[J]. Rare Metals, 2000, 19(2), 96-100.
[15]LIU Zeng-cai, LIN Le-yun, XU Jie, et al. Characteristics of original surface film of brass HSn70-1A and its corrosion behavior in seawater[J]. Chinese Journal of Rare Metals, 2000, 24(6): 466-469. (in Chinese)
Foundation item: Project (50271011) supported by the National Natural Science Foundation of China
Received date: 2004-03-17; Accepted date: 2004-07-01
Correspondence: ZHAO Yue-hong, PhD; Tel: +86-10-82241290; E-mail: lly1290@grinm.com


