
Vacuum distillation refining of metallurgical grade silicon (Ⅰ)——Thermodynamics on removal of phosphorus from metallurgical grade silicon
WEI Kui-xian(魏奎先)1, 2, MA Wen-hui(马文会)1, 2, DAI Yong-nian(戴永年)1, 2,
YANG Bin(杨 斌)1, 2, LIU Da-chun(刘大春)1, 2, WANG Jing-fu(汪镜福)1, 2
1. National Engineering Laboratory for Vacuum Metallurgy, Faculty of Materials and Metallurgical Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. Key Laboratory of Vacuum Metallurgy of Non-ferrous Metals of Yunnan Province, Kunming 650093, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
The removal of impurity phosphorus from metallurgical grade silicon is one of the major problems on purification of metallurgical grade silicon for solar grade silicon preparation. The thermodynamics on vacuum refining process of the metallurgical grade silicon was studied via separation coefficient of impurity phosphorus in the metallurgical grade silicon and vapor-liquid equilibrium composition diagram of Si-P binary alloy at different temperatures. The behaviors of impurity phosphorus in the vacuum distillation process were examined. The results show that the vacuum distillation should be taken to obtain silicon with less than 10-7 P, and the impurity phosphorus is volatilized easily by vacuum distillation in thermodynamics. Phosphorus is distilled from the molten silicon and concentrated in vapor phase.
Key words:
metallurgical grade silicon; solar grade silicon; refining; vacuum distillation; thermodynamics; phosphorus removal;
1 IntroductionSilicon has been widely used in the photovoltaic industry and semiconductor industry. Metallurgical grade silicon (MG-Si) is obtained from the carbothermic reduction of silica, containing some impurities such as Fe, Al, Ca and P, which affect the physiochemical properties of silicon materials[1]. In order to meet the requirement of solar grade silicon (SOG-Si), we should try to remove the impurities from metallurgical grade silicon.
Phosphorus is a typical impurity in silicon, and the required maximum limit for phosphorus content is 0.1×10-6 for SOG-Si[2]. Unfortunately, the segregation coefficient of phosphorus in silicon is large (0.35[3]), so it is difficult to remove phosphorus by zone or unidirectional solidification refining. The MG-Si can be refined by vacuum distillation[4-5], electron beam melting[1,6-8], solidification refining[9], and acid leach-ing treatment with addition of calcium[10], to meet the necessity of SOG-Si. In this study, the aim is to investigate systemically the thermodynamic performance for MG-Si refining via vacuum distillation to provide a theoretical base for SOG-Si production.
2 Relationship between vapor pressure of Si, P and temperatureBased on the different properties of elements contained when vaporizing and condensing, crude metal can be separated from impurities by vacuum distillation refining. The difference in vapor pressure of each metal at different temperatures is the basic principle of crude metal vacuum distillation. Relationship between the saturated pressure and temperature is shown in Eqn.(1), and the coefficients A, B, C and D for different substance are listed in Table 1.
Table 1 Values of coefficients of A, B, C and D for different substance[11]

lg p* =AT -1+BlgT+CT+D (1)
According to Eqn.(1) and Table 1, the relationship between saturated vapor pressure (p*, Pa) of phosphorus in the MG-Si and temperature (T, K) is obtained as
![]() (2)
(2)
![]() (3)
(3)
According to Eqns.(2) and (3), the saturated pressure of liquid of different substances can be worked out at a given temperature. Fig.1 presents the relationship between lg p* and T for phosphorus and silicon. As shown in Fig.1, phosphorus has a high saturated pressure with a normally low volatile rate, and silicon has low saturated pressure, which means it is difficult for silicon to volatile into gas phase from raw material at atmospheric pressure. However, under vacuum condition, the volatile rate will increase for both phosphorus and silicon. At the same temperature, the saturated pressure of phosphorus is much higher than that of silicon. At 1 732 K, silicon begins to melt and phosphorus sublime. The saturated pressure of phosphorus exceeds 108 Pa, which means that it would be distilled out of the silicon. As a result, theoretically, phosphorus and silicon can be separated by using vacuum distillation.

Fig.1 Relationship between saturated pressure and temperature for pure substance
3 Possibility of Si-P alloys separationAs the effect of one another between A and B binary alloys, the actual vapor pressure of A and B are not equal to theirs saturation vapor pressure. It must take into account the activity a and molar concentration N of A and B in A-B binary alloys if want to know the actual vapor pressure of A and B. The actual vapor pressures of A and B in A-B binary alloys are
![]() (4)
(4)
![]() (5)
(5)
where pA and pB are the actual vapor pressure of A and B, aA and aB are the activity of A and B, ![]() and
and ![]() are the saturation vapor pressure of A and B, NA and NB are the molar concentration of A and B, and gA and gB are the activity coefficients A and B, respectively.
are the saturation vapor pressure of A and B, NA and NB are the molar concentration of A and B, and gA and gB are the activity coefficients A and B, respectively.
According to the Eqns.(4) and (5), the following equation can be deduced as[12]:
![]() (6)
(6)
If
![]() (7)
(7)
Then
![]() (8)
(8)
bA is a function of the ratio of the concentrations of elements in the two phases, which is related to the activity coefficients and vapor pressures of the substances. We define bA as the separation coefficient of A, which can be used to determine whether element A separate from A-B binary alloys by vacuum distillation.
1) When bA=1, the composition of A and B in vapor and liquid phase are the same, A cannot be separated from B.
2) When bA>1, the composition A in vapor phase is more than in liquid phase, A can be separated from B. Composition A is concentrated in vapor phase, and composition B is concentrated in liquid phase. The bigger the value of bA is, the higher the removal efficiency will be.
3) When bA<1, the composition A in liquid phase is more than in vapor phase, A can be separated from B. Composition A is concentrated in liquid phase, and composition B is concentrated in vapor phase. The smaller the value of bA is, the higher the removal efficiency will be.
For P-Si binary alloy, bP>1, the content of composition P in vapor phase is more than in liquid phase, P can be separated from Si. Composition P is concentrated in vapor phase, and composition Si is concentrated in liquid phase.
According to Eqns.(2), (3) and (7), the separation coefficient of P calculated in the temperature range of 1 373-2 173 K is presented in Table 2.
Table 2 Variation of bP with temperature in binary alloy of Si-P
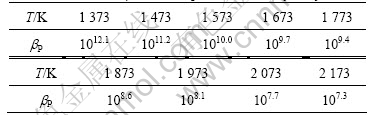
It can be seen from Table 2 that bP is much more than 1 (from 1 373 to 2 173 K). It can be concluded that P is concentrated into vapor phase and can be separated from Si completely.
4 Phase diagram of vapor-liquid equilibriumIt is important to estimate the separation effect and the products composition quantitatively. These data can be obtained by vapor-liquid equilibrium composition diagram, which was put forward in 1982. The purity of distillated MG-Si is predicted by vapor-liquid equilibrium composition diagram. For the binary alloy of Si-P, their contents in vapor and liquid are represented by xg(Si), xg(P), xl(Si), xl(P), respectively. We get
xg(Si)+xg(P) =1 (9)
xl(Si)+xl(P) = 1 (10)
The content of P in vapor is expressed as[13]
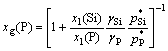
![]() (11)
(11)
Also, the content of Si in vapor is expressed as[13]
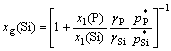 (12)
(12)
The relationship diagram of xg(P)-xl(P) can be developed by gSi, gP, ![]() and a series of xl(Si)/xl(P) at specific temperatures, that is the vapor-liquid equilibrium composition for Si-P system.
and a series of xl(Si)/xl(P) at specific temperatures, that is the vapor-liquid equilibrium composition for Si-P system.
In general, the MG-Si contains Si more than 98% and other impurities including Fe, Al, Ca and P and so on. The impurity phosphorus is less than 0.02% in purity. It is assumed that the impurity phosphorus can be considered the solute in dilute solution. The activity coefficient of Si is supposed as gSi = 1. As a result of the deficiency of activity coefficients of Si-P system, activity coefficients are identified based on the assumption that the highest and lowest mass ratios of impurities are 0.02% and 0.001%, respectively. Then according to Eqns.(2), (3), (11) and (12), the vapor-liquid phase equilibrium composition diagram of Si-P binary alloy system can be acquired as shown in Fig.2.
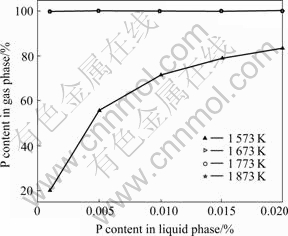
Fig.2 Vapor-liquid phase equilibrium composition diagram of Si-P alloy
Fig.2 illustrates that the proper temperature is in the range of 1 673-1 873 K to ensure evaporating loss of silicon, and silicon with less than 10-7 P can be obtained by vacuum distillation.
5 Conclusions1) The phosphorus impurity in the MG-Si can be easily removed via vacuum distillation in thermodynamics.
2) Vacuum distillation can be applied to produce silicon with less than 10-7 P in the temperature range of 1 373-2 173 K.
References[1] PIRES J C S, BRAGA A F B, MEI P R. Profile of impurities in polycrystalline silicon samples purified in an electron beam melting furnace [J]. Solar Energy Materials & Solar Cells, 2003, 79: 347- 355.
[2] DAVIS J R, ROHATGI A Jr, HOPKINS R H, BLAIS P D, RAI-CHOUDHURY P, MCCORMICK J R, MOLLENKOPF H C. Impurities in silicon solar cells [J]. Electron Devices, IEEE Transactions on Electron Devices, 1980, 27: 677-687.
[3] HOPKINS R H, ROHATGI A. Impurity effects in silicon for high efficiency solar cells [J]. J Cryst Growth, 1986, 75: 67-79.
[4] WEI Kui-xian, MA Wen-hui, DAI Yong-nian, YANG Bin, LIU Da-chun. Study on phosphorus removal from metallurgical grade silicon by vacuum distillation [J]. Acta Scientiarum Naturalium Universitatis Sunyatseni, 2007, 46(Suppl.): 69-71.
[5] SUZUKI K, SAKAGUCHI K, NAKAGIRI T, SANO N. Gaseous removal of phosphorus and boron from molten silicon [J]. J Japan Inst Metals, 1990, 54(2): 161-167. (in Japanese)
[6] YUGE N, ABE M, HANAZAWA K, BABA H, NAKAMURA N, KATO Y, SAKAGUCHI Y, HIWASA S, ARATANI F. Purification of metallurgical-grade silicon up to solar grade [J]. Progress in Photovoltaic: Research and Application, 2001, 9: 203-209.
[7] IKEDA T, MAEDA M. Purification of metallurgical silicon for solar grade silicon by electron beam button melting [J]. ISIJ International, 1992, 32 (5): 635-642.
[8] PIRES J C S, OTUBO J, BRAGA A F B, MEI P R. The purification of metallurgical grade silicon by electron beam melting [J]. Journal of Materials Processing Technology, 2005, 169: 16-20.
[9] TAKESHI Y, KAZUKI M. Removal of phosphorus by the solidification refining with Si-Al melts [J]. Science and Technology of Advanced Materials, 2003, 4: 531-537.
[10] SHIMPO T, YOSHIKAAWA T, MORITA K. Thermodynamic study of the effect of calcium on removal of phosphorus from silicon by acid leaching treatment [J]. Metall Mater Trans B, 2004, 35: 277-284.
[11] KUBASCHEWSKI O, ALCOCK C B. Metallurgical Thermo- chemistry [M]. Beijing: Metallurgical Industry Press, 1985: 486-513. (in Chinese)
[12] DAI Yong-nian, ZHAO Zhong. Vacuum metallurgy [M]. Beijing: Metallurgical Industry Press, 1988: 114-115. (in Chinese)
[13] DAI Yong-nian, YANG Bin. Vacuum metallurgy of non-ferrous metals [M]. Beijing: Metallurgical Industry Press, 2000: 55-57. (in Chinese)
(Edited by YUAN Sai-qian)
Foundation items: Project(50674050) supported by the National Natural Science Foundation of China; Project(2006BAE01B08) supported by Sustentation Project of Science and Technology of China; Project(20060674004) supported by the Doctorial Programs Foundation of Ministry of Education of China.
Corresponding author: MA Wen-hui; E-mail: mwhui@kmust.edu.cn.


