DOI: 10.11817/j.ysxb.1004.0609.2020-37530
氰化尾渣中金铁梯级提取及铁精粉中杂质形成机理
傅平丰1, 2,王化军1, 2,胡文韬1, 2,边振忠1,李振宇1
(1. 北京科技大学 土木与资源工程学院, 北京 100083;
2. 金属矿山高效开采与安全教育部重点实验室, 北京 100083)
摘 要:采用“磁化焙烧-硫脲浸金-磁选-碱浸除杂”的金铁梯级提取法从焙烧氰化尾渣中浸出金,并制取铁精粉,通过物相转化、焙烧过程热力学计算和颗粒群结构分析,揭示铁精粉中杂质形成机理。结果表明:氰化尾渣添加8%焦粉于700 ℃下磁化焙烧60 min,焙烧样以硫脲法浸金,金浸出率达65.87%;浸金渣经磨矿磁选得到TFe品位为55.01%的初级铁精粉,再于90 ℃的10%NaOH溶液中碱浸8 h,可得TFe品位为62.22%、回收率为69.80%的合格铁精粉。物相转化和热力学计算表明,磁化焙烧过程中含铁矿物与Si、Ca、Al及重金属等杂质反应,生成铁橄榄石、钙铝榴石和铁钙辉石等新物相,与磁铁矿紧密共生,混入铁精粉中;微细粒磁铁矿存在严重磁团聚,石英等杂质会机械夹杂在磁团聚中,降低铁精粉质量。
关键词:
焙烧氰化尾渣;梯级提取;磁化焙烧;铁精粉;碱浸;物相转化;热力学计算;
文章编号:1004-0609(2020)-03-0666-09 中图分类号:X756 文献标志码:A
焙烧氰化尾渣是金精矿经硫酸化焙烧-氰化提金后产生的固体废物,由其粒度细、外观呈红褐色且为粉末状,俗称“红渣”。我国已连续10多年是全球最大黄金生产国,每年排放的焙烧氰化尾渣已达几百万吨。因含氰化物及砷、铅等重金属,堆存氰化尾渣不仅占用大量土地,还污染地表和地下水系,给生态环境和居民健康带来严重危害[1-2]。氰化尾渣已被列为危险废物(无机氰化固体废物,代码092-003-33),需严格按照危险废物处置要求进行管理和处置,因此,如何有效处理氰化尾渣,已成为各黄金冶炼企业亟需解决的问题。
焙烧氰化尾渣中主要有价金属为金、银和铁,金品位多为1~3 g/t,部分尾渣金品位可达7 g/t以上,TFe品位约30%,主要为赤铁矿,含有少量磁铁矿和黄铁矿[2]。当前,对焙烧氰化尾渣中金银回用的研究较多,但缺少对含铁资源高效利用的研究[3-4]。利用氰化尾渣制备铁精粉或铁红颜料,可回收铁资源,降低二次尾渣量,消除氰化物危害。磁化焙烧法可将尾渣中赤铁矿还原成磁铁矿,磁选制取铁精粉,过程简单、易于工业化生产,已有不少此类报道[5-6]。但研究表明,由于焙烧氰化尾渣含有大量Si、Ca、Al及重金属等杂质,尾渣在磁化焙烧过程中,矿物的物相转化和反应过程远比天然赤褐铁矿复杂,铁精粉TFe品位低(约52%~58%),杂质含量高,难以达到炼铁工业标准[5-7]。添加钠盐或CaO的磁化焙烧,改变氰化尾渣组分,可提高铁精粉质量。ZHANG等[8]添加3%碳酸钠和10%硫酸钠磁化焙烧,水浸后磁选,可得TFe品位59.11%的铁精粉;张亚莉等[7]添加CaO使尾渣中n(CaO):n(SiO2)为1:1,磁化焙烧-磁选后可得TFe品位60%的铁精粉。但是添加大量无机盐或CaO进行磁化焙烧,增加处理成本及增大二次渣量,且铁精粉TFe品位提高幅度不大。目前,针对氰化尾渣磁化焙烧法制得的铁精粉TFe品位低、杂质多的问题,仍缺少系统性机理研究。
本研究采用“磁化焙烧-硫脲浸金-磁选-碱浸除杂”的梯级金铁提取工艺,以焙烧氰化尾渣为原料,通过磁化焙烧-硫脲浸出回收金,浸金渣经弱磁选制取TFe品位约55%的初级铁精粉,再碱浸除杂,溶去铁精粉中Si、Al及重金属等杂质,最终获取TFe品位>62%的合格铁精粉。以SEM-EDS、XRD和激光粒度法分析焙烧样和铁精粉的颗粒群结构、物相组成和粒度特性,计算磁化焙烧过程中主要矿物的反应热力学,揭示以焙烧氰化尾渣为原料,磁化焙烧制取铁精粉杂质含量高的机理。
1 实验
1.1 实验原料
实验所用氰化尾渣来自河南某黄金冶炼厂排放的焙烧氰化尾渣,外观为红褐色,呈粉末状。氰化尾渣的化学组成见表1,其TFe品位为27.51%,金品位为3.4 g/t,是可回收的有价金属;但尾渣中Si、S和As等有害杂质含量较高,且含少量Pb、Zn等重金属。图1所示的氰化尾渣物相组成表明,主要矿物为赤铁矿和石英,还有少量二水石膏和海蓝石(Gartrellite, Pb(Cu,Fe)2(AsO4,SO4)2(CO3,H2O)0.7, PDF 46-1306)。尾渣的粒度组成如图2所示,其平均粒径为7.91 μm,80%的颗粒小于13.14 μm。以上分析表明,焙烧氰化尾渣具有粒度细、组成复杂、杂质含量高的特点,有价金属回收难度大。尾渣磁化焙烧的焦粉取自山东聊城,研细后其粒度为<0.074 mm的占76.10%(质量分数),焦粉的干燥基工业分析结果见表2,其固定碳含量达83.82%(质量分数)。初级铁精粉碱浸除杂所用NaOH为分析纯,碱浸用水为去离子水。
表1 焙烧氰化尾渣的化学组成
Table 1 Chemical composition of roasting cyanide tailings (mass fraction,%)
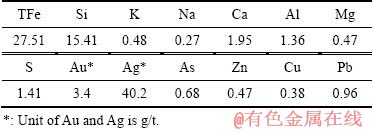
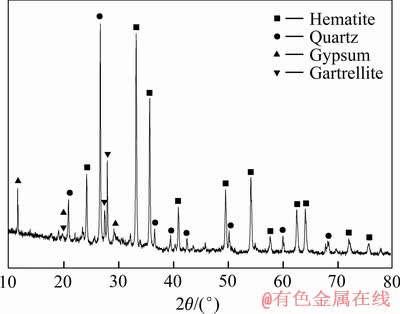
图1 焙烧氰化尾渣的XRD谱
Fig. 1 XRD pattern of roasting cyanide tailings
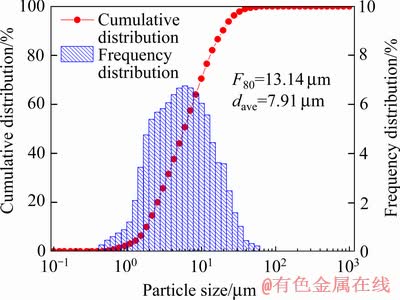
图2 焙烧氰化尾渣的粒度组成分析
Fig. 2 Particle size distribution analysis of roasting cyanide tailings
表2 焦粉的干燥基工业分析结果
Table 2 Drying proximate quality analysis of coke powders (mass fraction,%)
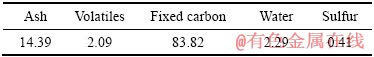
1.2 磁化焙烧条件实验
焙烧氰化尾渣的磁化焙烧于CD-1400X型马弗炉中完成,将30 g氰化尾渣与焦粉按一定比例混匀,焦粉用量为3%~20%(焦粉质量占氰化尾渣质量分数),混合料置于石墨坩埚中,当炉内温度升高到设定值后,将石墨坩埚放入马弗炉中磁化焙烧,达到预定焙烧时间后,取出热焙烧样并迅速水淬冷却。焙烧样用自来水按3:1的液固比配制浸金矿浆,FeCl3用量25 kg/t,硫脲用量30 kg/t,调整矿浆pH值到1.5,用JJ-1型电动搅拌机搅拌浸出金,浸出温度40 ℃,浸出时间10 h,搅拌速率400 r/min,硫脲浸出后过滤得到浸金渣和含金贵液。测定含金贵液中金和银含量,计算金和银的浸出率。将浸金渣配水调成矿浆,用CXG-99型磁选管进行一段磁选,磁场强度选为79.6 kA/m,将磁选精矿和尾矿分别过滤、烘干、化验TFe品位,计算出精矿的铁回收率。
1.3 磁选流程优化实验
确定磁化焙烧条件后,将浸金渣按图3所示的磨矿和磁选流程优化选别工艺参数,因氰化尾渣经焙烧后有结块出现,采用一段磨矿、二段弱磁选流程制取铁精粉。磨矿细度为<0.038 mm的占87.66%,一、二段磁选的磁场强度分别为218.8 kA/m和198.9 kA/m,获得初级铁精粉。
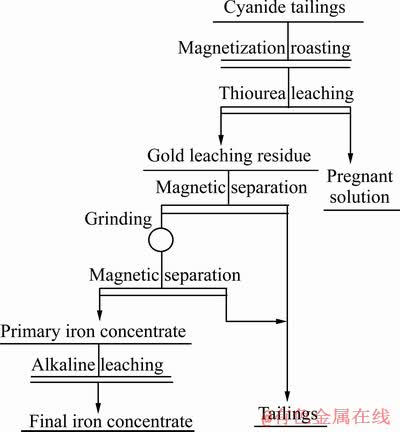
图3 焙烧氰化尾渣梯级回收金铁的工艺流程图
Fig. 3 Flowsheet of cascading recovery of gold and iron from roasting cyanide tailings
1.4 初级铁精粉碱浸除杂实验
将15 g初级铁精粉放入锥形瓶中,加入10%~ 20%(质量分数)的NaOH溶液60 mL,碱浸液固比为4:1,将锥形瓶固定于水浴中,用JJ-1型电动搅拌机搅拌浸出,搅拌速率400 r/min,碱浸时间8 h,浸出温度80~90 ℃,浸出后经过滤、烘干得到合格铁精粉。
1.5 检测分析
X射线衍射(XRD)采用日本理学Rigaku-RA型X射线衍射仪测定,扫描范围10°<2θ<90°,扫描速率6 (°)/min。扫描电镜-能谱分析(SEM-EDS)采用德国卡尔蔡司EVO18型扫描电子显微镜,放大倍数5~50万倍,加速电压200 V~30 kV,分析前将粉末样品固定于导电胶带上,再粘附于样品台,表面喷炭以增强其导电性。激光粒度分析采用日本株式会社清新企业生产的LMS-30型激光粒度仪,测定范围0.1~1000 μm,颗粒样品分散介质为无水乙醇,分析前样品于超声波振荡分散器中分散5 min。X射线荧光分析(XRF)采用日本岛津公司的EDX8000型X射线荧光光谱仪做定量分析,配备硅漂移检测器(SDD),检测元素含量范围为0.0001%~99.99%。样品中TFe含量和氰化尾渣中Au品位由化学分析测得,浸金贵液中金含量采用TAS-990型原子吸收分光光度计测定。
2 结果与讨论
2.1 磁化焙烧影响因素
2.1.1 焙烧温度的影响
当焦粉用量10%、焙烧时间为60 min时,焙烧温度对焙烧样的金浸出率和铁精矿分选指标的影响如图4所示,当温度升高到700~750 ℃时,焙烧样的金浸出率达64%左右,说明在磁化焙烧过程中,随着赤铁矿的还原及其晶格受热应力作用,被包裹于赤铁矿晶格中的金已部分暴露,能被硫脲浸出;但当焙烧温度升高到800 ℃以上,焙烧样变得结构致密,出现结块现象,降低金浸出率,此与生成低熔点的渣相有关[9]。随着焙烧温度升高,精矿的TFe回收率也增加,表明尾渣中赤铁矿已逐步还原成磁铁矿,700 ℃焙烧所得精矿的TFe品位达52.36%。赤铁矿颗粒在磁化焙烧过程中由外及里按Fe2O3→Fe3O4→ FeO顺序被还原,FeO的生成与还原气氛中CO浓度相关,温度上升会提高气相中CO浓度,促进FeO相生成,故过高的焙烧温度会导致磁铁矿过还原[10-11]。磁选结果表明,当焙烧温度超过750 ℃时,精矿TFe回收率大幅降低,与生成弱磁性FeO相物质有关。当焙烧温度从600 ℃增加900 ℃,精矿TFe品位不断降低,表明随着含铁矿物被还原,尾渣中SiO2、CaO、Al2O3等杂质组分经固相反应生成了新杂质相,与磁铁矿紧密共生(或团聚),磁选过程中被富集于精矿中。
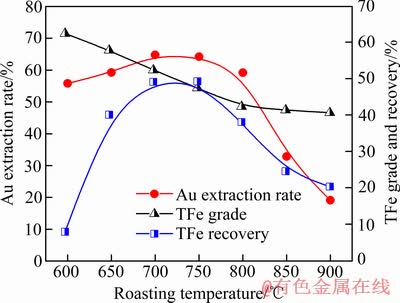
图4 焙烧温度对金浸出率和精矿TFe品位与回收率的影响
Fig. 4 Effect of roasting temperature on Au extraction rate, TFe grade and recovery of iron concentrates
2.1.2 焙烧时间的影响
当焦粉用量10%、焙烧温度为700 ℃时,焙烧时间对焙烧样的金浸出率和精矿分选指标的影响如图5所示,当焙烧时间从15 min增加到60 min,金浸出率从28.37%提高到64.75%,延长焙烧时间促进尾渣中金的暴露,可提高金的浸出率。但是,当焙烧时间延长到120 min时,金浸出率却有所下降,此与长时间焙烧致使焙烧料的结构变得致密有关。当焙烧时间为60 min时,精矿的TFe回收率最高,表明60 min焙烧可使尾渣中赤铁矿基本被还原成磁铁矿。进一步延长焙烧时间导致TFe回收率下降,与含铁矿物的过还原有关,将磁铁矿还原成FeO相,降低磁性,并形成低熔点化合物[11-12]。
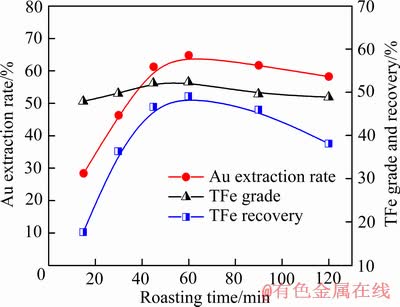
图5 焙烧时间对金浸出率和精矿的TFe品位与回收率的 影响
Fig. 5 Effect of roasting time on Au extraction rate, TFe grade and recovery of iron concentrates
2.1.3 焦粉用量的影响
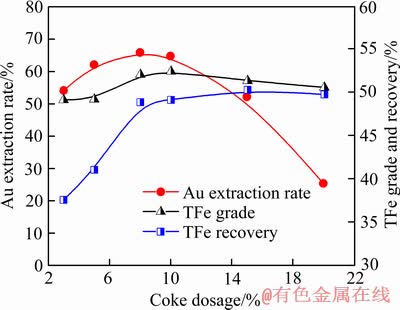
图6 焦粉用量对金浸出率和精矿的TFe品位与回收率影响
Fig. 6 Effect of coke powder dosage on Au extraction rate, TFe grade and recovery of iron concentrates
当焙烧温度为700 ℃、焙烧时间为60 min时,焦粉用量对焙烧样的金浸出率和精矿分选指标的影响如图6所示,随着焦粉用量从3%增加到8%,金浸出率从54.12%提高65.87%,说明尾渣中赤铁矿还原,增加包裹金的暴露率,提高金浸出率。但是,当焦粉用量增加至20%时,金浸出率大幅下降至25.31%。实验观察到残留有未反应的焦粉,因焦粉吸附浸出的金,导致很低的金浸出率。当焦粉用量增至8%~10%时,TFe回收率上升到49%左右,精矿TFe品位约为52%。当焦粉用量超过10%,TFe品位略有下降,还原剂过量会导致气相中CO浓度过高,磁铁矿会被过还原生成FeO相(见式(1)),降低TFe品位[10-11, 13]。综合考虑金浸出率和精矿的TFe品位及回收率,确定磁化焙烧条件为焙烧温度700 ℃、焙烧时间60 min和焦粉用量8%。此条件下,焙烧样中银的浸出率达54.27%。
Fe3O4+CO(g)=3FeO+CO2(g) (1)
2.2 磁选流程优化和铁精粉碱浸除杂
采用图3所示的一段磨矿、二段磁选流程,优化磨矿、磁选工艺参数,可得TFe品位55.01%、回收率72.49%的初级铁精粉,其化学组成如表3所列,可见,初级铁精粉的Si、Ca、Al及重金属等杂质含量较高。磁选尾矿的产率为62.71%,因已经700 ℃磁化焙烧,原始尾渣中残留的氰化浸出剂可被高温分解,所以与原始氰化尾渣相比,磁选尾矿的毒性降低,同时磁选尾矿的总量也大幅减小。
已有研究表明NaOH溶液可与SiO2[14]、Al2O3[14]、氧化砷[15]、铅锌氧化物[16-18]等杂质反应,将其转化成水溶性盐(见式(2)~(6))。因此,针对初级铁精粉中Si、Al及重金属等杂质含量较高的问题,采用NaOH碱浸法去除初级铁精粉中杂质,设计4组碱浸实验(条件1:浸出温度80 ℃+10%NaOH;条件2:浸出温度80 ℃+ 20%NaOH;条件3:浸出温度90 ℃+10%NaOH;条件4:浸出温度90 ℃+20%NaOH),碱浸结果如图7所示。由图7可见,碱浸能使铁精粉TFe品位提高5%以上,当浸出温度90 ℃和NaOH浓度10%时,碱浸所得的合格铁精粉TFe品位达62.22%,碱浸过程TFe回收率为96.29%,换算成全流程TFe回收率为69.80%。合格铁精粉的化学组分如表3所列,与初级铁精粉相比,TFe品位提高7.21%,Si、Al、As、Pb和Zn等杂质含量明显降低。
表3 初级铁精粉和合格铁精粉的化学组成
Table 3 Chemical compositions of primary and qualified iron concentrates (ICs)
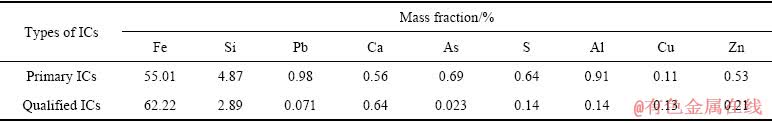
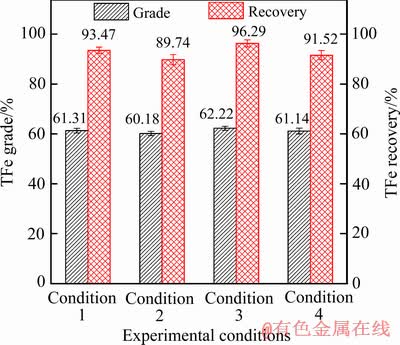
图7 初级铁精粉NaOH碱浸试验结果
Fig. 7 Alkaline leaching of primary iron concentrates in NaOH solutions
nSiO2+2NaOH=Na2O·nSiO2+H2O (2)
Al2O3+2NaOH+3H2O=2NaAl(OH)4 (3)
As2O3+2NaOH=2NaAsO2+H2O (4)
ZnO+2NaOH=Na2ZnO2+H2O (5)
PbO+2NaOH=Na2PbO2+H2O (6)
以上结果表明,焙烧氰化尾渣经“磁化焙烧-硫脲浸金-磁选-碱浸除杂”的梯级金铁提取工艺处理,金和银的浸出率分别达65.87%和54.27%,获得的合格铁精粉TFe品位62.22%、铁回收率达69.80%,实现了氰化尾渣的资源化利用,并同步达到危险固废的无害化和减量化的目的。与国内以磁铁矿为主的C60级铁精矿质量标准相比,以焙烧氰化尾渣制取的铁精粉,其TFe品位和SiO2、S、Cu、Pb、Zn和As等有害杂质含量均能满足C60级铁精矿的质量要求,由于其粒度较细,可用作生产球团矿的原料,为焙烧氰化尾渣中铁资源的规模化利用提供了新 途径。
2.3 铁精粉中杂质形成的机理分析
2.3.1 焙烧过程中物相转化分析
图8所示为不同温度下磁化焙烧样的XRD谱。与图1对比可见,当焙烧温度为600 ℃时,部分赤铁矿已被还原成磁铁矿。随着焙烧温度升高,赤铁矿被还原的速率增加,当焙烧温度为700 ℃时,赤铁矿基本被还原成磁铁矿,与较高TFe回收率(见图4)相一致。当焙烧温度升高到800 ℃时,焙烧样XRD谱虽未出现FeO相的衍射峰,但精矿TFe回收率却下降到38.05%(见图4),表明磁铁矿颗粒表层或有部分区域被还原成FeO,形成Fe3O4+FeO的Fe-O平衡体系[19]。当温度继续升高到900 ℃时,磁铁矿的衍射峰强度已很低,出现大量铁橄榄石(Fe2SiO4)衍射峰,说明高温还原时氰化尾渣中SiO2与含铁矿物反应生成了铁橄榄石,同时,尾渣中CaO、Al2O3、SiO2会与FeO相反应,生成了钙铝榴石(Ca3Al2Si3O12)、铁钙辉石(CaFeSi2O6),特别是铁钙辉石在800 ℃焙烧时已有明显衍射峰。可见,在磁化焙烧过程中,因焙烧氰化尾渣含有大量CaO、Al2O3和SiO2等杂质,当还原体系中有FeO相生成时,生成了弱磁性的铁橄榄石、铁钙辉石等新杂质相,此类物质与磁铁矿紧密共生,磁选过程中被富集于精矿中,造成初级铁精粉TFe品位低、杂质含量高。
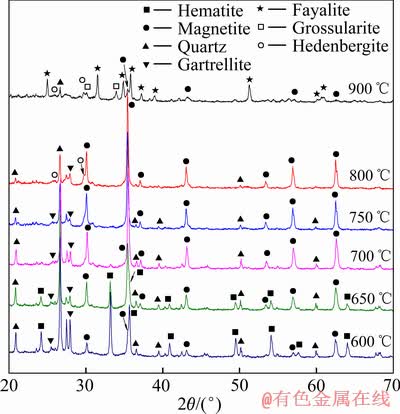
图8 不同温度下磁化焙烧样的XRD谱
Fig. 8 XRD patterns of magnetization roasting samples obtained at different temperatures
2.3.2 焙烧过程中主要反应的热力学分析
焙烧氰化尾渣中赤铁矿在含CO的气氛中被还原时,可与石英反应生成Fe2SiO4(见式(7)),随反应温度升高,此反应的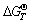 负值的绝对值不断增大(见图9),说明升高温度能促进铁橄榄石生成。工业氰化浸金需加入CaO调整矿浆pH值,故尾渣中CaO含量较高,CaO与Fe2SiO4生成CaSiO3(见式(8)),能将铁橄榄石中FeO置换出来。因此,若提高CaO含量,可抑制铁橄榄石生成,张亚莉等[7]发现将氰化尾渣中m(CaO):m(SiO2 )提高到1:1,可提高铁精粉TFe品位与回收率,但本研究中氰化尾渣的Ca含量只有1.95%,远低于Si含量(15.14%),不足以抑制铁橄榄石的生成。氰化尾渣中CaO还能与FeO、SiO2和Al2O3反应,生成CaFeSi2O6(见式(9))和Ca3Al2Si3O12(见式(10)),此二种物质已出现在焙烧样中(见图8)[20]。此外,焙烧过程中CaO易与SiO2生成Ca2SiO4(见式(11)),Ca2SiO4再与Fe2O3反应可生成CaFe2O4(见式(12)),此反应的
负值的绝对值不断增大(见图9),说明升高温度能促进铁橄榄石生成。工业氰化浸金需加入CaO调整矿浆pH值,故尾渣中CaO含量较高,CaO与Fe2SiO4生成CaSiO3(见式(8)),能将铁橄榄石中FeO置换出来。因此,若提高CaO含量,可抑制铁橄榄石生成,张亚莉等[7]发现将氰化尾渣中m(CaO):m(SiO2 )提高到1:1,可提高铁精粉TFe品位与回收率,但本研究中氰化尾渣的Ca含量只有1.95%,远低于Si含量(15.14%),不足以抑制铁橄榄石的生成。氰化尾渣中CaO还能与FeO、SiO2和Al2O3反应,生成CaFeSi2O6(见式(9))和Ca3Al2Si3O12(见式(10)),此二种物质已出现在焙烧样中(见图8)[20]。此外,焙烧过程中CaO易与SiO2生成Ca2SiO4(见式(11)),Ca2SiO4再与Fe2O3反应可生成CaFe2O4(见式(12)),此反应的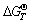 随焙烧温度升高负值绝对值不断增大,说明高温还原焙烧易生成CaFe2O4。另外,焙烧氰化尾渣中含有一定量ZnO、CuO和MgO,此类氧化物与Fe2O3反应可生成类Fe3O4结构的尖晶石(XFe2O4),由图9可见,式(13)~(15)的
随焙烧温度升高负值绝对值不断增大,说明高温还原焙烧易生成CaFe2O4。另外,焙烧氰化尾渣中含有一定量ZnO、CuO和MgO,此类氧化物与Fe2O3反应可生成类Fe3O4结构的尖晶石(XFe2O4),由图9可见,式(13)~(15)的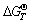 在磁化焙烧温度范围内均<0 kJ/mol,说明能生成含Mg、Zn和Cu的尖晶石。其实,XFe2O4尖晶石中X所代表的Fe2+、Mg2+、Zn2+和Cu2+离子半径相近(见表4),固相反应过程中可取代Fe3O4中Fe2+离子,产生类质同象效应,生成结构与Fe3O4相似的尖晶石[21]。通过热力学分析可知,焙烧氰化尾渣中Si、Ca、Al及重金属等杂质在焙烧过程中能生成新杂质相,与Fe3O4紧密共生,磁选时混入精矿,降低初级铁精粉质量。
在磁化焙烧温度范围内均<0 kJ/mol,说明能生成含Mg、Zn和Cu的尖晶石。其实,XFe2O4尖晶石中X所代表的Fe2+、Mg2+、Zn2+和Cu2+离子半径相近(见表4),固相反应过程中可取代Fe3O4中Fe2+离子,产生类质同象效应,生成结构与Fe3O4相似的尖晶石[21]。通过热力学分析可知,焙烧氰化尾渣中Si、Ca、Al及重金属等杂质在焙烧过程中能生成新杂质相,与Fe3O4紧密共生,磁选时混入精矿,降低初级铁精粉质量。
Fe2O3+SiO2+CO=Fe2SiO4+CO2 (7)
CaO+Fe2SiO4=CaSiO3+2FeO (8)
CaO+FeO+2SiO2=CaFeSi2O6 (9)
3CaO+Al2O3+3SiO2=Ca3Al2Si3O12 (10)
SiO2+2CaO=Ca2SiO4 (11)
Ca2SiO4+2Fe2O3=2CaFe2O4+SiO2 (12)
ZnO+Fe2O3=ZnFe2O4 (13)
CuO+Fe2O3=CuFe2O4 (14)
MgO+Fe2O3=MgFe2O4 (15)
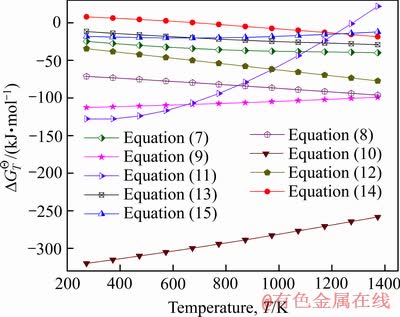
图9 式(7)~(15)的标准自由能变化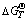 与温度T的关系
与温度T的关系
Fig. 9 Relationship of Gibbs free energy changes  with temperature T for equations (7)-(15)
with temperature T for equations (7)-(15)
表4 Fe2+、Mg2+、Zn2+和Cu2+的离子半径
Table 4 Ionic radius of Fe2+, Mg2+, Zn2+ and Cu2+ ions ( )
)

2.3.3 初级铁精粉的颗粒群结构分析
图10所示为初级铁精粉的SEM像,由图10(a)可见,铁精粉中有大量结构疏松、直径>20 μm的团聚体,增加放大倍数(见图10(b)),团聚体主要由直径5~10 μm的大颗粒和大量<2 μm的微细颗粒组成,微细颗粒附着于大颗粒表面或填充于大颗粒之间的空隙中,形成较为稳定的团聚体。初级铁精粉的粒度组成如图11所示,其平均粒径达23.05 μm,与图10(a) 中观察到的团聚体直径>20 μm相一致,但远高于焙烧氰化尾渣的平均粒径7.91 μm(见图2),表明微细粒磁铁矿颗粒在磁选过程被磁化,因受颗粒间磁力作用,产生严重的磁团聚。磁团聚的形成增加了微细粒磁铁矿被弱磁选回收的可能,但细粒石英、铁橄榄石和铁钙辉石等杂质能以机械夹杂的形式混入磁团聚中,增加铁精粉杂质含量。图10(c)的能谱分析表明,图10 (b)中沉积于大颗粒表面的小颗粒(“+”)主要由Si、Ca、O组成,说明有细粒杂质矿物粘附于磁铁矿颗粒表面,夹杂于磁团聚体中。
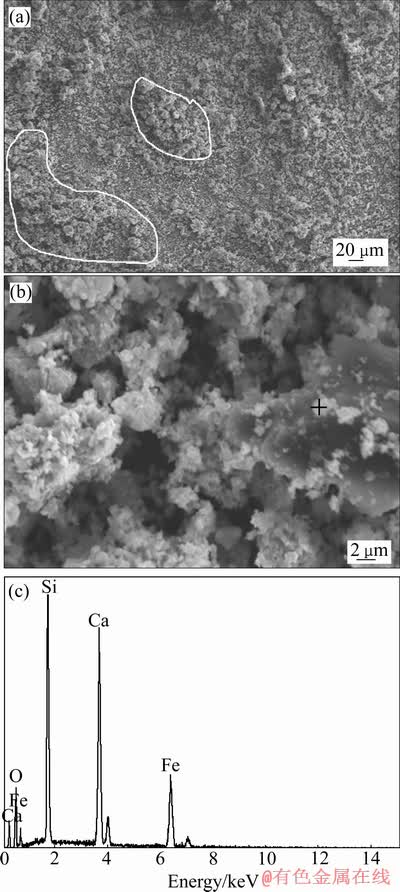
图10 初级铁精粉的SEM像和EDS谱
Fig. 10 SEM images((a), (b)) and EDS spectrum(c) of primary iron concentrates
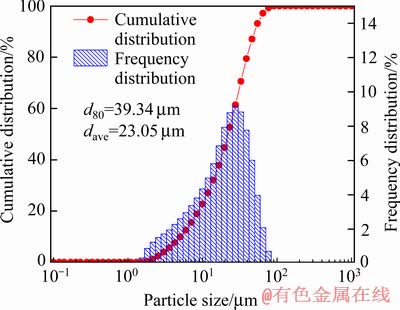
图11 初级铁精粉的粒度组成分析
Fig. 11 Particle size distribution of primary iron concentrates
3 结论
1) 以微细粒焙烧氰化尾渣为原料,采用“磁化焙烧-硫脲浸金-磁选-碱浸除杂”的梯级金铁提取工艺浸出金及制取铁精粉,添加8%焦粉后,混合料于700 ℃下磁化焙烧60 min,焙烧样用硫脲法浸金,金和银的浸出率分别达65.87%和54.27%;浸金渣经一段磨矿、二段弱磁选,得到TFe品位55.01%、回收率72.49%的初级铁精粉;初级铁精粉以NaOH碱浸除杂,得到TFe品位62.22%、回收率69.80%的合格铁精粉,碱浸能大幅降低铁精粉中Si、Al、As、Pb和Zn等杂质含量。
2) 物相转化分析和热力学计算表明,焙烧氰化尾渣中Si、Ca、Al及重金属等杂质在磁化焙烧过程中易生成铁橄榄石、钙铝榴石、铁钙辉石等新杂质相,因新生杂质与磁铁矿紧密共生,磁选时混入精矿,降低初级铁精粉质量。
3) 还原生成的磁铁矿在磁选时形成磁团聚,虽能提高微细粒磁铁矿被弱磁选回收的可能性,但细粒石英、铁钙辉石等杂质能以机械夹杂的形式混入磁团聚中,增加铁精粉杂质含量。
REFERENCES
[1] DONATO D B, NICHOLS O, POSSINGHAM H, MOORE M, RICCI P F, NOLLER B N. A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife[J]. Environment International, 2007, 33(7): 974-984.
[2] 边振忠, 傅平丰, 李振宇. 焙烧氰化尾渣中金、银和铁的回收利用研究进展[J]. 贵金属, 2017, 38(3): 88-92.
BIAN Zhen-zhong, FU Ping-feng, LI Zhen-yu. Research progress of recycling gold, silver and iron from roasted cyanide tailings[J]. Precious Metals, 2017, 38(3): 88-92.
[3] 郑雅杰, 龚 昶, 孙召明. 氰化尾渣还原焙烧酸浸提铁及氰化浸金新工艺[J]. 中国有色金属学报, 2014, 24(9): 2426-2433.
ZHENG Ya-jie, GONG Chang, SUN Zhao-ming. New technology of iron extraction and gold recovery from cyanide tailings by cyanide process after reduction roasting and acid leaching[J]. The Chinese Journal of Nonferrous Metals, 2014, 24(9): 2426-2433.
[4] FU Ping-feng, LI Zhen-yu, FENG Jie, BIAN Zhen-zheng. Recovery of gold and iron from cyanide tailings with a combined direct reduction roasting and leaching process[J]. Metals, 2018, 8(7): 561.
[5] 谢建宏, 张崇辉, 李 慧, 刘思伟, 王 素. 某焙烧氰化尾渣综合利用试验研究[J]. 金属矿山, 2011(1): 150-152.
XIE Jian-hong, ZHANG Chong-hui, LI Hui, LIU Si-wei, WANG Su. Experimental researches on comprehensive utilization of roasting cyanided tailings[J]. Metal Mine, 2011(1): 150-152.
[6] 尚德兴, 陈芳芳, 张亦飞, 曾 鸣. 还原焙烧-磁选回收氰化尾渣中铁的试验研究[J]. 矿冶工程, 2011, 31(5): 35-38.
SHANG De-xing, CHEN Fang-fang, ZHANG Yi-fei, ZENG Ming. Recovery of iron from gold-cyanide residue by reduction roasting and magnetic separation[J]. Mining and Metallurgical Engineering, 2011, 31(5): 35-38.
[7] 张亚莉, 于先进, 李小斌, 张丽鹏, 李德刚. 氰化渣磁化焙烧过程中铁化合物反应行为的热力学分析[J]. 中南大学学报(自然科学版), 2011, 42(12): 3623-3629.
ZHANG Ya-li, YU Xian-jin, LI Xiao-bin, ZHANG Li-peng, LI De-gang. Thermodynamics analysis of ferric compound during roasting-preparing process of cyanide tailings[J]. Journal of Central South University (Science and Technology), 2011, 42(12): 3623-3629.
[8] ZHANG Y L, LI H M, YU X J. Recovery of iron from cyanide tailings with reduction roasting-water leaching followed by magnetic separation[J]. Journal of Hazardous Materials, 2012, 213/214: 167-174.
[9] LIU B L, ZHANG Z H, LI L B, WANG Y J. Recovery of gold and iron from the cyanide tailings by magnetic roasting[J]. Rare Metal Materials and Engineering, 2013, 42(9): 1805-1809.
[10] GUO X, SASAKI Y, KASHIWAYA Y, ISHII K. Microreaction mechanism in reduction of magnetite to wustite[J]. Metallurgical and Materials Transactions B, 2004, 35(3): 517-522.
[11] LEI C, YAN B, CHEN T, XIAO X M. Recovery of metals from the roasted lead-zinc tailings by magnetizing roasting followed by magnetic separation[J]. Journal of Cleaner Production, 2017, 158: 73-80.
[12] PENG T F, GAO X C, LI Q B, XU L J, LUO L Q, XU L H. Phase transformation during roasting process and magnetic beneficiation of oolitic-iron ores[J]. Vacuum, 2017, 146: 63-73.
[13] 耿 超, 孙体昌, 杨慧芬, 马友文, 胡天洋. 添加剂对海滨钛磁铁矿直接还原磁选钛铁分离的影响[J]. 中国有色金属学报, 2017, 27(8): 1720-1728.
GENG Chao, SUN Ti-chang, YANG Hui-feng, MA You-wen, HU Tian-yang. Effect of additives on titanium and iron separation from beach titanomagnetite by direct reduction followed by magnetic separation[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(8): 1720-1728.
[14] WANG R C, ZHAI Y C, NING Z Q, MA P H. Kinetics of SiO2 leaching from Al2O3 extracted slag of fly ash with sodium hydroxide solution[J]. Transactions of Nonferrous Metals Society of China, 2014, 24(6): 1928-1936.
[15] 吴玉林, 徐志峰, 郝士涛. 炼铜烟灰碱浸脱砷的热力学及动力学[J]. 有色金属(冶炼部分), 2013(4): 3-7.
WU Yu-lin, XU Zhi-feng, HAO Shi-tao. Thermodynamics and kinetics of alkaline leaching of arsenic in copper smelting slags[J]. Nonferrous Metals (Extractive Metallurgy), 2013(4): 3-7.
[16] ORHAN G. Leaching and cementation of heavy metals from electric arc furnace dust in alkaline medium[J]. Hydrometallurgy, 2005, 78: 236-245.
[17] 乔晋玺, 龙 双, 马雅琳, 邱 洋, 陈敬阳, 苗华磊, 陈爱良. 空气氧化碱浸含砷钴镍渣[J]. 中国有色金属学报, 2018, 28(11): 2358-2365.
QIAO Jin-xi, LONG Shuang, MA Ya-lin, QIU Yang, CHEN Jing-yang, MIAO Hua-lei, CHEN Ai-liang. Alkali leaching of cobalt/nickel residue containing arsenic using air[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(11): 2358-2365.
[18] 郭学益, 江晓健, 刘静欣, 刘 旸, 刘子康. 梯级碱溶分步提取废弃电路板中有价金属[J]. 中国有色金属学报, 2017, 27(2): 406-413.
GUO Xue-yi, JIANG Xiao-jian, LIU Jing-xin, LIU Yang, LIU Zi-kang. Recovery of metal values from waste printed circuit boards using a cascading alkali leaching process[J]. The Chinese Journal of Nonferrous Metals, 2017, 27(2): 406-413.
[19] LI J, LI B, HAN J, GAO Z, WANG J. A comparative study on the reduction mechanism of Fe2O3 under different heating methods[J]. JOM, 2014, 66(8): 1529-1536.
[20] 张林楠, 张 力, 王明玉. 高铁CaO-FeOx-SiO2 三元体系氧化过程相变热力学分析[J]. 物理化学学报, 2008, 24(9): 1540-1546.
ZHANG Lin-nan, ZHANG Li, WANG Ming-yu. Thermodynamics of phase transformations in oxidation process of CaO-FeOx-SiO2 system with high iron content[J]. Acta Physico-Chimica Sinica, 2008, 24(9): 1540-1546.
[21] COSTA R, LELIS M, OLIVEIRA L, FABRIS J, ARDISSON J, RIOS R, SILVA C. Novel active heterogeneous Fenton system based on Fe3-xMxO4(Fe, Co, Mn, Ni): The role of M2+ species on the reactivity towards H2O2 reactions[J]. Journal of Hazardous Materials, 2006, 129(1/3): 171-178.
Cascading recovery of gold and iron from cyanide tailing and formation mechanisms of impurities in iron concentrates
FU Ping-feng1, 2, WANG Hua-jun1, 2, HU Wen-tao1, 2, BIAN Zhen-zhong1, LI Zhen-yu1
(1. School of Civil and Resources Engineering, University of Science and Technology Beijing, Beijing 100083, China;
2. State Key Laboratory of High-Efficient Mining and Safety of Metal Mines, Ministry of Education, Beijing 100083, China)
Abstract: A cascading process, namely, magnetization roasting-thiourea leaching-magnetic separation-alkaline leaching, was developed to extract gold and prepare iron concentrates (ICs) from roasting cyanide tailing (RCT). The formation mechanisms of impurities in ICs were revealed by phase transformation, thermodynamics calculation and structure analysis. The results show that, after magnetization roasting of RCT by adding 8% coke powders at 700 ℃ for 60 min, Au extraction rate of 65.87% is achieved by thiourea leaching. Rough ICs with TFe grade of 55.01% are obtained by grinding and magnetic separation of leaching residue. By further leached in 10% NaOH solution, qualified ICs with TFe grade of 62.22% and recovery of 69.80% are prepared. The phase transformation and thermodynamics calculation reveal that fayalite, grossular and hedenbergite, tightly combing with magnetite, are generated by reacting iron oxides with Si, Al, Ca and heavy metals. Structural analysis exhibits that the impurity minerals are mechanically mixed into formed magnetic agglomerates, resulting in low quality of ICs.
Key words: roasting cyanide tailing; cascading recovery; magnetization roasting; iron concentrates; alkaline leaching; phase transformation; thermodynamics calculations
Foundation item: Project(2018YFC1900604) supported by National Key Research and Development Program of China; Projects(51674017, 51874017) supported by National Natural Science Foundation of China
Received date: 2019-03-18; Accepted date: 2019-06-24
Corresponding author: FU Ping-feng; Tel: +86-13520202167; E-mail: pffu@ces.ustb.edu.cn
(编辑 李艳红)
基金项目:国家重点研究发展计划资助项目(2018YFC1900604);国家自然科学基金资助项目(51674017,51874017)
收稿日期:2019-03-18;修订日期:2019-06-24
通信作者:傅平丰,副教授,博士;电话:13520202167;E-mail:pffu@ces.ustb.edu.cn
[2] 边振忠, 傅平丰, 李振宇. 焙烧氰化尾渣中金、银和铁的回收利用研究进展[J]. 贵金属, 2017, 38(3): 88-92.
[3] 郑雅杰, 龚 昶, 孙召明. 氰化尾渣还原焙烧酸浸提铁及氰化浸金新工艺[J]. 中国有色金属学报, 2014, 24(9): 2426-2433.
[5] 谢建宏, 张崇辉, 李 慧, 刘思伟, 王 素. 某焙烧氰化尾渣综合利用试验研究[J]. 金属矿山, 2011(1): 150-152.
[6] 尚德兴, 陈芳芳, 张亦飞, 曾 鸣. 还原焙烧-磁选回收氰化尾渣中铁的试验研究[J]. 矿冶工程, 2011, 31(5): 35-38.
[7] 张亚莉, 于先进, 李小斌, 张丽鹏, 李德刚. 氰化渣磁化焙烧过程中铁化合物反应行为的热力学分析[J]. 中南大学学报(自然科学版), 2011, 42(12): 3623-3629.
[13] 耿 超, 孙体昌, 杨慧芬, 马友文, 胡天洋. 添加剂对海滨钛磁铁矿直接还原磁选钛铁分离的影响[J]. 中国有色金属学报, 2017, 27(8): 1720-1728.
[15] 吴玉林, 徐志峰, 郝士涛. 炼铜烟灰碱浸脱砷的热力学及动力学[J]. 有色金属(冶炼部分), 2013(4): 3-7.
[17] 乔晋玺, 龙 双, 马雅琳, 邱 洋, 陈敬阳, 苗华磊, 陈爱良. 空气氧化碱浸含砷钴镍渣[J]. 中国有色金属学报, 2018, 28(11): 2358-2365.
[18] 郭学益, 江晓健, 刘静欣, 刘 旸, 刘子康. 梯级碱溶分步提取废弃电路板中有价金属[J]. 中国有色金属学报, 2017, 27(2): 406-413.
[20] 张林楠, 张 力, 王明玉. 高铁CaO-FeOx-SiO2 三元体系氧化过程相变热力学分析[J]. 物理化学学报, 2008, 24(9): 1540-1546.


