
Effect of N-substituents on performance of thiourea collectors
by density functional theory calculations
LIU Guang-yi(刘广义), ZHONG Hong(钟 宏), XIA Liu-yin(夏柳荫),
WANG Shuai(王 帅), DAI Ta-gen(戴塔根)
Institute of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China
Received 2 April 2009; accepted 31 May 2009
Abstract:
Using density functional methods, some properties were studied such as the energies and compositions of frontier molecular orbitals and the atomic charges, which are related to the reactive behavior of thioureas containing different N-substituent groupings. The calculation results indicate that the N-substituent groupings have significant effect on the flotation performance of thiourea collectors. The order of electron-donating ability is N-propyl-N′-benzyl-thiourea (PBZYTU)>N-propyl-N′-ethyl-thiourea (PETU) >N-propyl-N′-allyl-thiourea (PALTU)>> N-propyl-N′-acetyl-thiourea (PACTU) >N-propyl-N′-ethoxycarbonyl- thiourea (PECTU) >N-propyl-N′-benzoyl-thiourea (PBZOYTU), and the order of feedback-electron-accepting ability is PBZOYTU>PACTU>PECTU>> PALTU>PETU>PBZYTU. This implies that PBZOYTU, PACTU or PECTU can react with copper atoms having (t2g)6(eg)3Cu(II) or t6e4Cu(I) configuration on the surfaces of copper sulfide minerals through normal covalent bond and back donation covalent bond, and exhibit excellently collecting performance for copper sulfide minerals. These are consistent with the experimental data reported in the literatures.
Key words:
thiourea collector; sulfide ore; structure-activity relationship; density functional method;
1 Introduction
Collector study is a crucial part of flotation development, since ores usually consist of a mixture of minerals, and success of the separation process depends mainly on the selective adsorption of the collector. Since the choice of collectors type is crucial in the flotation performance of minerals, the research for new chemical reagents having strong affinity and better selectivity for certain metal ions has attracted much attention recently. A selective adsorption of the collector can be achieved through an understanding of the properties of the mineral surface and collectors, and corresponding surface reactions.
Xanthates can strongly interact with the majority of sulfide minerals to form metal xanthate and dixanthogen, however, the selectivity is low without the use of additional reagents. In flotation separation of copper sulfide minerals from iron sulfide minerals, for example, lime is used to maintain pH values over 10.5, more usually above 11.0 and often as high as 12.0 or 12.5[1] or 13.0[2]. Thionocarbamates (TCs) are more selective for copper sulfide against gangue iron sulfides, it shows superior metallurgical performance compared with xanthates under alkaline and neutral pH values usually below 11.5[2-9]. Thiourea or substituted thiourea also shows superior metallurgical performance in recovering valued metals from their ores by froth flotation[10]. Recently, ethoxycarbonyl thioureas (TUs) have been proved to be superior collectors compared with ethoxycarbonyl TCs[1, 5, 11-12]. Whereas, the flotation performances of TU collectors containing different N-substituent groupings have seldom been mentioned, and systemically theoretical investigation on the structure-reactivity relationship of TU collectors has not been done in the previous studies.
Density functional theory (DFT), offered an effective tool in the calculation of some properties and energies of the various collectors[13-16]. Solvent effects affect the molecular structure, mechanism of chemical reactions in solution, etc. Physical and chemical properties such as geometry of molecules, charge distribution and reactivity in solution often vary from those in vacuum[17]. In the present study, vacuum and solution DFT calculations were employed to investigate the influence of N-substituent on the efficiencies of TU, namely, N-propyl-N′-ethyl-thiourea (PETU), N-propyl- N′-benzyl-thiourea (PBZYTU), N-propyl-N′-allyl- thiourea (PALTU), N-propyl-N′-ethoxycarbonyl-thiourea (PECTU), N-propyl-N′-benzoyl-thiourea (PBZOYTU), N-propyl-N′-acetyl-thiourea (PACTU) as selective collectors for copper sulfide minerals in flotation process. Thus, the structural information, relative energies, atomic charges, highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies and compositions about TU containing different N-substituent groupings were obtained by the density functional theory computations. The calculated properties of the free molecules are the useful information for better understanding the interaction and bond formation between the chelating molecule and the mineral surface.
2 Method
All calculations were made using the Gaussian03 [18] and Chemoffice2005 program. The initial molecular modeling of PETU, PALTU, PBZYTU, PACTU, PBZOYTU and PECTU were optimized by MM2 (a modified version of Allinger’s MM2 force field) and MP3 (Parameterized Model revision 3) methods. The obtained geometries were further optimized and calculated with Becke’s three-parameter hybrid exchange functional and Lee-Yang-Parr correlation functional (B3LYP). We calculated the partial charge on each atom by Mulliken population analysis (MPA) and natural population analysis (NPA) with 6-31g(d) split-valence basis set. For investigation of electronic structure of TU molecule in solution, we used the integral equation formalism for the polarizable continuum model (IEF-PCM)[19] in DFT calculations. The adopted value of dielectronic constant for water was 78.39.
3 Theoretical background
The perturbation energy of the approaching two reactants collector and mineral may be approximated as [9, 20-21]
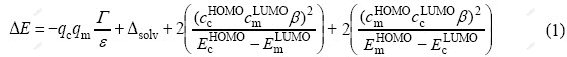
where qc and qm are total initial charges, Γ is the coulomb repulsion term, ε is the local dielectric constant of solvent, Δsolv is the desolvation energy, HOMO is the highest occupied molecular orbit, LUMO is the lowest unoccupied molecular orbit, ccHOMO and ccLUMO are the frontier orbital electron densities, β is the extent of bonding in transition state, and EcHOMO–EmLUMO is the energy difference of frontier orbitals. When |EcHOMO– EmLUMO| is large, very little charge transfer occurs; the reaction is primarily determined by the total charges on the reactants (charge-controlled reaction). On the other hand, when the two frontier orbitals are nearly degenerate, i.e. |EcHOMO–EmLUMO|≈0, their interaction (electron transfer) becomes significant. This frontier- orbital-controlled reaction is enhanced by high polarizability and low solvation energies, and it leads to a covalent bonding and can be associated with soft-soft interaction.
Eq.(1) is the essential basis for the chemical reaction between flotation reagents and minerals. The energy is composed of electrostatic effect, solution effect, normal covalent bond, and back donation covalent bond orderly. The energy of electrostatic effect is proportional to the net charge at the reactive center of flotation reagent or mineral. The energy of normal covalent bond depends on the electron-donating power of reagent and electron-accepting power of mineral. The energy of back donation covalent bond is related to electron-back- donating power of mineral and electron-accepting power of reagent. The chemical reaction between sulfide mineral and its collector is a frontier-controlled reaction, thereby making the normal covalent bond and back donation covalent bond especially important in assessing the flotation performance of collector for sulfide ore.
4 Results and discussion
4.1 Surface properties of copper and iron sulfide minerals
The chalcopyrite band gap was 0.6 eV measured by CRUNDWELL[22], and the value of pyrite band gap was reported as 0.9 eV by BULLETT[23]. This means that electrons in occupied d-orbitals of copper atom on chalcopyrite surface are more easily transferring to unoccupied orbitals of collector than that of iron atom on pyrite surface.
Under the alkaline conditions, the surface oxidation layer of pyrite consists only of iron(Ⅲ) oxyhydroxide (probably goethite) , and there is no detectable FeS2 remaining[24-25], and the oxidation products of chalcopyrite surfaces are mostly iron(Ⅲ) oxyhydroxide, leaving Cu and S unoxidized in the original chalcopyrite structure as a metastable phase of CuS2 stoichiometry [24,26]. As a result, on the surfaces of copper sulfide minerals, the copper having (t2g)6(eg)3Cu(Ⅱ or t6e4Cu(Ⅰ) configuration can accept frontier electron of collectors to form a σ-bond, and also easily donates its richly d-orbital electron (feedback electron) to the frontier unoccupied orbital of collector resulting in the formation of a dative π-bond. While, on the surfaces of iron sulfide minerals, the iron having (t2g)6(eg)0Fe(Ⅱ) and (t2gа)3(egа)2 Fe(Ⅲ) configuration (Fe(Ⅲ) is the predominant composition at alkaline conditions) interacts chemically with collectors basically through accepting frontier electron of collectors to form a σ-bond.
4.2 Electronic structures of TU collectors
The optimized collector geometries with the numbering schema at B3LYP/6-31g(d) level are shown in Fig.1. The selected optimized geometric parameters of studied molecules are given in Table 1.
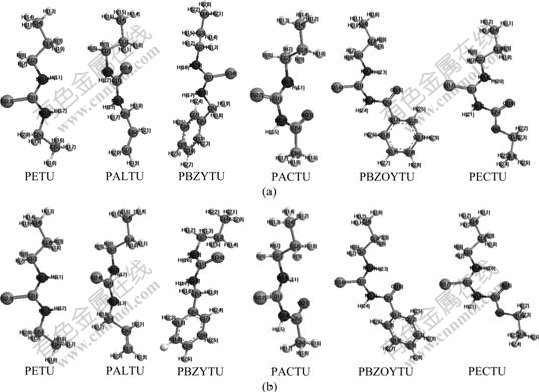
Fig.1 Optimized geometries of TU molecules at B3LYP/6-31g(d) level: (a) In vacuum; (b) In solution
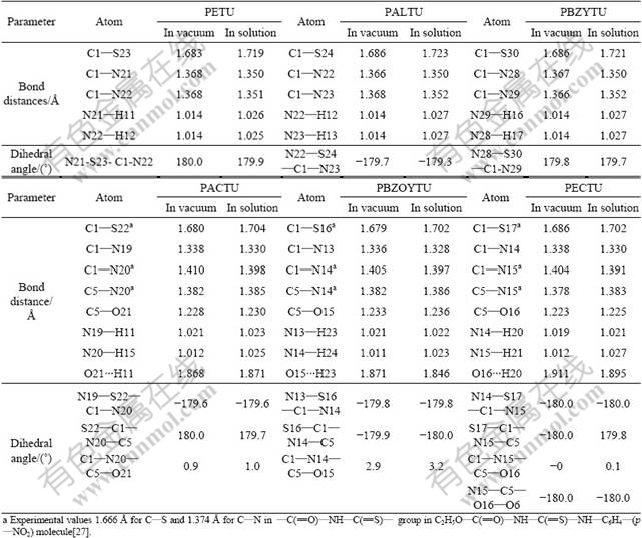
The solution DFT calculations indicate that compared with (1.721±0.002) ? in PETU, PALTU and PBZYTU molecules, the C=S bond distances decrease to (1.703±0.001) ? (Table 1) in PACTU, PBZOYTU and PECTU molecules with a gap of 0.018 ?, which inferred that the C=S double bond is stronger in PACTU, PBZOYTU and PECTU than in PETU, PALTU and PBZYTU. As a result, the S atom in PACTU, PBZOYTU and PECTU molecules is more difficult to lose its electrons. Table 1 also indicates that the C=S bond distance in solution is greater than that in vacuum, which implies the sulfur atom in C=S group is more active reactivity in aqueous solution. The selected dihedral angles in Table 1 are nearly 180?, which means every atom in —N—C(=S)—N— group in PETU, PALTU and PBZYTU molecules or every atom in —C(=O)—N—C(=S)—N— group in PACTU, PBZOYTU and PECTU molecules almost own one plane. This is a key for formation of conjugated π- or π*-bond, based on FMO theory.
Table 1 Selected optimized geometric parameters of studied molecules
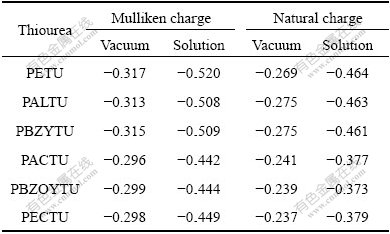
The net charges of sulfur atom in TU molecules with DFT calculations at B3LYP/6-31g(d) level are given in Table 2. The results of solution DFT calculations indicate that the Mulliken charge of sulfur atom is followed in this order: PACTU>PBZOYTU>PECTU>> PALTU>PBZYTU>PETU. The electronic charge of sulfur atom in C=S group is more negative in solution than in that in vacuum. This means the TU molecule may improve its electrostatic affinity with mineral surface in polar aqueous solution.
Table 2 Net charge (in electron) of sulfur atom in TU molecules with B3LYP/6-31g(d) calculations
The frontier orbital eigenvalues and compositions of collectors are given in Table 3. Table 3 demonstrates that the HOMOs of PACTU, PBZOYTU, PECTU, PALTU, PBZYTU and PETU are composed of 3p orbitals of sulfur atoms in each collector molecule, which infers that the sulfur atom is of the reactive center of these collectors and can donate its frontier electron to metal atom on the mineral surface resulting in the formation of σ-bond. The third unoccupied molecular orbitals (TUMO) of PBZYTU and the LUMO of PACTU, PBZOYTU, PECTU, PALTU and PETU indicate that these colloctors had the power of overlapping of d-orbitals of metal atom with their FMO to form dative π-bond. Table 3 also indicates that the frontier orbital eigenvalues of TU molecule were more negative in solution than those in vacuum. This might be owed to the influence of solvent effects on the properties of TU molecule. Water slightly changes the reactivity of TU molecule through Van der Waals force, hydrogen bonding or electrostatic attraction.
4.3 Structure-activity relationships for TU collectors
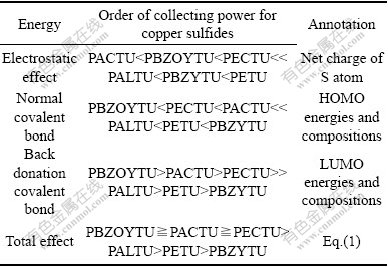
GLEMBOTSKII[28] suggested that the collecting power of thio collectors is directly proportional to the electron density of the reactive center of the molecule (i.e., sulfur). The electronic charge of sulfur atom (Table 2) is followed in this order: PACTU>PBZOYTU>PECTU>> PALTU>PBZYTU>PETU. Hence, based on the electrostatic effect, the collecting power of these collectors for copper and iron sulfide minerals is followed as PACTU<PBZOYTU<PECTU<<PALTU<PBZYTU<PETU.
The effectiveness of a molecule may be related to the certain quantum-chemical parameters. Among these, we want to mention the energy of the HOMO that is often associated with the capacity of a molecule to donate the electrons. The HOMO and LUMO orbitals are commonly known as the frontier orbitals and were found to be extremely useful in explaining chemical reactivity. Since chemical bonds are mostly the product of the valence electrons, and the spatial distribution of these electrons is determined by the HOMO orbital, electrophilic attacks can be correlated very well with atomic sites having high density of the HOMO orbital. As the title compounds act as electron donor species, the HOMO orbitals were especially taken into account in this study.
Table 3 Frontier orbital eigenvalues and compositions of TU molecules with DFT calculations at B3LYP/6-31g(d) level
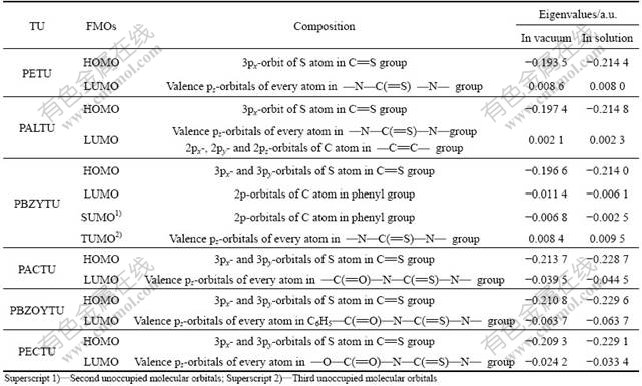
The HOMO energies (Table 3) of the compounds increase in the following order: PBZYTU>PETU> PALTU>>PACTU>PECTU>PBZOYTU (in aqueous solution). The higher HOMO energy level means that the molecule easily releases the electrons to the unoccupied orbitals of the metal atom, and it has better activity used as collector. Accordingly, based on the normal covalent bond (denominator term) in Eq.(1), the collecting power of these collectors for copper and iron sulfide minerals is followed as PBZYTU>PETU>PALTU>>PACTU> PECTU>PBZOYTU.
The TUMO energy of PBZYTU and the LUMO energies of PACTU, PBZOYTU, PECTU, PALTU and PETU increase in the following order: PBZOYTU< PACTU<><><>
The LUMO of PETU is constituted by valence pz-orbitals of every atom in —N—C(=S)—N— group and could form back donation covalent bond by overlapping with the d-orbitals of the copper atom on the mineral surface. While the four-membered chelate ring formed between PETU and metal atom would be unstable. The TUMO of PBZYTU is also constituted by valence pz-orbitals of every atom in —N—C(=S)—N— group and could form back donation covalent bond by overlapping with the d-orbitals of the copper atom. Besides valence pz-orbitals of every atom in —N—C(=S)—N— group, 2pz-orbitals of C atom in —C=C— group in PALTU molecule could also overlap with d-orbitals of the copper atom, which could enhance the collecting activity of PALTU.
The LUMOs of PACTU, PECTU and PBZOYTU were constituted by valence pz-orbitals of every atom in the —C(=O)—N—C(=S)—N— group, which was a conjugated π*-bond that would readily delocalize electrons and form back donation covalent bond by overlapping with the d-orbitals of the copper atom on the mineral surface. This would enhance the degree of overlapping by the copper and sulfur orbitals, thereby enhancing the total bond strength. As a result, it is suggested that during the interaction between PECTU (or PACTU, and/or PBZOYTU) and copper atom on the surfaces of copper sulfide minerals, PECTU (or PACTU, and/or PBZOYTU) offers its HOMO electrons of thiocarbonyl sulfur atom to copper atom, forming σ-bond, and simultaneously, copper atom donates its d-orbital electrons to the LUMO of PECTU (or PACTU, and/or PBZOYTU), forming dative π-bond. PECTU (or PACTU, and/or PBZOYTU) should react chemically with copper atom on the surface of copper sulfide minerals to form a stably six/five membered complex, thereby making PECTU, PACTU and PBZOYTU more powerful collectors for copper sulfide minerals than PBZYTU, PETU and PALTU.
Based on above discussion, the effect of N-substituents on flotation performance of TU collectors is summarized in Table 4. It can be seen from Table 4 that PECTU, PACTU and PBZOYTU are stronger collectors for copper sulfide minerals than PBZYTU, PETU and PALTU since the ethylcarbonyl and acetyl groups are electron-withdrawing, while the ethyl, allyl and phenyl groups are weakly electron-donating. The electron-withdrawing substituents in N-alkyl groupings reduce the electrostatic effect and normal covalent bond, thereby reducing the reactive strength between sulfide minerals and collectors, and improving the selectivity of collectors against iron sulfide minerals. While the ethylcarbonyl and acetyl groups are adjacent to —N—C(=S)—N— group, which can form a conjugated π*-bond that readily delocalizes electrons and overlaps richly d-orbital electrons. Chalcopyrite has a narrow band gap and the copper atoms with (t2g)6(eg)3Cu(Ⅱ) and t6e4Cu(Ⅰ) configuration on its surface, thereby making the back donation covalent bond stronger, while pyrite having a broad band gap and (t2g)6(eg)0Fe(Ⅱ) and (t2gа)3(egа)2 Fe(Ⅲ) on its surfaces acts in the opposite manner. Hence, compared with PBZYTU, PETU and PALTU, PECTU, PACTU and PBZOYTU collectors are more powerful for copper sulfide minerals and more selective against iron sulfide minerals. The previous studies have proved that the ethoxycarbonyl thioureas are excellent collectors for the flotation separation of Cu/Fe sulphide minerals[1, 5, 11-12]. Because thionocarbamates are popular collectors for the flotation of sulfide minerals, we think thioureas should be worthy of doing more study in the future.
Table 4 Effect of N-substituents on flotation performance of TU collectors
5 Conclusions
1) From the HOMO energies and the electron density of the reactive center, the following collecting ability order is theoretically obtained: PBZOYTU< PECTU<><><> 2) Concerning the LUMO energies and compositions of collectors and the HOMO compositions of metal atoms on mineral surfaces, the collecting power of the collectors for copper sulfide minerals is followed as PBZOYTU > PACTU > PECTU >> PALTU > PETU > PBZYTU. It is concluded that PECTU, PACTU or PBZOYTU has a better performance as flotation collector with improved collecting power for copper sulfide minerals and selectivity against iron sulfide minerals than PBZYTU, PETU or PALTU. 3) As a result, this study provides a straightforward use of the computational techniques for the description of the activities of the chemical systems. References [1] FU Y L, WANG S S. Neutral hydrocarboxycarbonyl thiourea sulfide collectors [P]. US Patent RE32786, 1988-11-22. [2] LIU G Y, ZHONG H, DAI T G. The separation of Cu/Fe sulfide minerals at slightly alkaline conditions by using ethoxycarbonyl thionocarbamates as collectors: Theory and practice [J]. Minerals Engineering, 2006, 19(13): 1380-1384 [3] WOODS R, HOPE G A. A SERS spectroelectrochemical investigation of the interaction of O-isopropyl-N- ethylthionocarbamate with copper surfaces [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 1999, 146(1/3): 63-74. [4] FAIRTHORNE G, FORNASIERO D, RALSTON J. Solution properties of thionocarbamate collectors [J]. International Journal of Mineral Processing, 1996, 46(1/2): 137-153. [5] FAIRTHORNE G, FORNASIERO D, RALSTON J. Interaction of thionocarbamate and thiourea collectors with sulfide minerals: a flotation and adsorption study [J]. International Journal of Mineral Processing, 1997, 50(4): 227-242. [6] SHEN W Z, FORNASIERO D, RALSTON J. Effect of collectors, conditioning pH and gases in the separation of sphalerite from pyrite [J]. Minerals Engineering, 1998, 11(2): 145-158. [7] BOULTON A, FORNASIERO D, RALSTON J. Depression of iron sulfide flotation in zinc roughers [J]. Minerals Engineering, 2000, 14(9): 1067-1079. [8] NAGARAJ D R, BRINEN J S. SIMS study of adsorption of collectors on pyrite [J]. International Journal of Mineral Processing, 2001, 63(1): 45-57. [9] LIU G Y, ZHONG H, DAI T, XIA L Y. Investigation of the effect of N-substituents on performance of thionocarbamates as selective collectors for copper sulfides by ab initio calculations [J]. Minerals Engineering, 2008, 21(12/14): 1050-1054. [10] PETROVICH V. Froth flotation method for recovering metal values from their ores by thiourea or substituted thiourea [P]. US Patent 4256227, 1981. [11] FAIRTHORNE G, BRINEN J S, FORNASIERO D, NAGARAJ R, RALSTON J. Spectroscopic and electrokinetic study of the adsorption of butyl ethoxycarbonyl thiourea on chalcopyrite [J]. International Journal of Mineral Processing, 1998, 54(3/4): 147-163. [12] LIU Guang-yi, ZHONG Hong, DAI Ta-gen, XIA Liu-yin. Studies on ethoxycarbonyl thiourea collectors in the flotation separation of Cu/Fe sulfide minerals under middle alkaline conditions: Principle and practice [J]. The Chinese Journal of Nonferrous Metals, 2009, 19(2): 389-395, (in Chinese) [13] PORENTO M, HIRVA P. Theoretical studies on the interaction of anionic collectors with Cu+, Cu2+, Zn2+ and Pb2+ ions [J]. Theoretical Chemistry Accounts: Theory, Computation and Modeling, 2002, 107: 200-205. [14] PORENTO M, HIRVA P. A theoretical study on the interaction of sulfhydryl surfactants with a covellite (001) surface [J]. Surface Science, 2004, 555(1/3): 75-82. [15] YEKELER M, YEKELER H. Reactivities of some thiol collectors and their interactions with Ag+ ion by molecular modeling [J]. Applied Surface Science, 2004, 236(1/4): 435-443. [16] YEKELER M, YEKELER H. A density functional study on the efficiencies of 2-mercaptobenzoxazole and its derivatives as chelating agents in flotation processes [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2006, 286(1/3): 121-125. [17] ABRONIN I A, BURSHTEIN K Y, ZHIDOMIROV G M. Quantum-chemical calculations of solvent effects on the electronic structure and reactivity of molecules [J]. Journal of Structural Chemistry, 1980, 21(2): 145-164. [18] FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 03 Revision B.01 [M], Pittsburgh PA, USA: Gaussian, Inc., 2003. [19] CHIPMAN D M. Reaction field treatment of charge penetration [J]. Journal of Chemical Physics, 2000, 112(13): 5558-5565. [20] KLOPMAN G. Chemical reactivity and paths [J]. Journal of American Chemical Society, 1968, 90(2): 223-230. [21] WANG D, LIN Q, JIANG Y. Molecular design of reagents for mineral and metallurgical processing [M]. Changsha: Central South University of Technology Press, 1996: 88-110. [22] CRUNDWELL F K. The influence of the electronic structure of solids on the anodic dissolution and leaching of semiconducting sulphide minerals [J]. Hydrometallurgy, 1988, 21(2): 155-190. [23] BILLETT D W. Electronic structure of 3d pyrite- and arcasite-type sulphides [J]. Journal of Physics C: Solid State Physics, 1982, 15: 6163-6174. [24] VAUGHAN D J, BECKER U, WRIGHT K. Sulphide mineral surfaces: Theory and experiment [J]. International Journal of Mineral Processing, 1997, 51(1/4): 1-14. [25] TODD E C, SHERMAN D M, PURTON J A. Surface oxidation of pyrite under ambient atmospheric and aqueous (pH=2 to 10) conditions: Electronic structure and mineralogy from X-ray absorption spectroscopy [J]. Geochimica et Cosmochimica Acta, 2003, 67(5): 881-893. [26] TODD E C, SHERMAN D M, PURTON J A. Surface oxidation of chalcopyrite (CuFeS2) under ambient atmospheric and aqueous (pH 2-10) conditions: Cu, Fe L- and O K-edge X-ray spectroscopy [J]. Geochimica et Cosmochimica Acta, 2003, 67(12): 2137-2146. [27] SHEN X, SHI X F, KANG B S, TONG Y X, LIU Y, GU L Q, LIU Q T, HUANG Y Y. Preparation and crystal structure of a new Cu(Ⅱ) complex derived from the desulfurization of N-(p-nitrophenyl)-N- ethoxycarbonyl-thiourea [J]. Polyhedron, 1999, 18(1/2): 33-37. [28] GLEMBOTSKII A V. Theoretical principles of forecasting and modifying collector properties [J]. Tsvet Metal, 1977, 50(4): 61-65. Foundation item: Project(50604016) supported by the National Natural Science Foundation of China; Project(2007B52) supported by the Foundation for the Author of National Excellent Doctoral Dissertation of China; Project(NCET-08-0568) supported by the Program for New Century Excellent Talents in Chinese University; Project(2007CB613602) supported by the National Basic Research Program of China; Project(2007AA06Z122) supported by the National High-tech Research and Development Program of China; Project(2007BAB22B01) supported by the National Science and Technology Support Project of China Corresponding author: LIU Guang-yi; Tel: +86-731-88830603; E-mail: guangyi.liu@163.com DOI: 10.1016/S1003-6326(09)60200-4
Abstract: Using density functional methods, some properties were studied such as the energies and compositions of frontier molecular orbitals and the atomic charges, which are related to the reactive behavior of thioureas containing different N-substituent groupings. The calculation results indicate that the N-substituent groupings have significant effect on the flotation performance of thiourea collectors. The order of electron-donating ability is N-propyl-N′-benzyl-thiourea (PBZYTU)>N-propyl-N′-ethyl-thiourea (PETU) >N-propyl-N′-allyl-thiourea (PALTU)>> N-propyl-N′-acetyl-thiourea (PACTU) >N-propyl-N′-ethoxycarbonyl- thiourea (PECTU) >N-propyl-N′-benzoyl-thiourea (PBZOYTU), and the order of feedback-electron-accepting ability is PBZOYTU>PACTU>PECTU>> PALTU>PETU>PBZYTU. This implies that PBZOYTU, PACTU or PECTU can react with copper atoms having (t2g)6(eg)3Cu(II) or t6e4Cu(I) configuration on the surfaces of copper sulfide minerals through normal covalent bond and back donation covalent bond, and exhibit excellently collecting performance for copper sulfide minerals. These are consistent with the experimental data reported in the literatures.
Journal of Chemical Physics, 2000, 112(13): 5558-5565." target="blank">[19] CHIPMAN D M. Reaction field treatment of charge penetration [J]. Journal of Chemical Physics, 2000, 112(13): 5558-5565.


