
Steady polarization process modelling of noble metal-electrolyte cermet composite electrode
LUO Zhi-an(罗志安), XIAO Jian-zhong(肖建中), XIA Feng(夏 风), YANG Yi-fan(杨一凡)
State Key Lab of Plastic Forming Simulation and Die and Mould Technology,
Huazhong University of Science and Technology, Wuhan 430074, China
Received 30 May 2005; accepted 26 September 2005
Abstract:
Cermet composites containing mixture of noble metal phase and electrolyte phase are the state-of-the-art electrode materials used for electrochemical sensor and solid oxide fuel cell(SOFC). A steady polarization model was developed. The model was based on electronic and ionic transfer process together with the electrochemical reaction regardless of mass transport in the electrode. The modelling results can help to understand the electrochemistry of cermet composite electrode.
Key words:
noble metal; cermet composite electrode; steady polarization; modelling; zirconia;
1 Introduction
In recent years, the extensive researches on the fuel cells and electrochemical sensors have by a long way promoted the development of cermet composite electrode composed of metal and electrolyte[1-3]. The electrochemically active component of oxygen sensor is a typical system of this kind of electrode. The sensor consists of three main parts: the anode, the electrolyte and the cathode. Even though the cermet electrodes are of great interest as central part of the sensor, the electrochemical reaction mechanism is not yet well understood[4, 5]. This is mainly due to three reasons. Firstly, the cermet electrode made of platinum and yttria stabilized zirconia, i.e. Pt-YSZ, has a complex microstructure with three interconnecting and interpenetrating network of metal Pt particles, YSZ electrolyte particles and pores. Thus, it is difficult to characterize and quantify the area where the electrochemical reaction takes place[6]. Secondly, the results obtained from the main characterization techniques, such as electrochemical impedance spectroscopy(EIS) and electrode polarization curve, are difficult to well interpret the reaction process in electrochemical or physical way[7]. Thirdly, because of the limitation of in-situ detection, some fundamental surface science data are not available under particularly rigorous conditions in operation. Therefore, there is no widely accepted theory on the function and effect of composition and microstructure of the composite electrode[8]. So, in this paper, the aim is to investigate the reaction mechanism of the cermet composite electrode. It is expected to facilitate a better understanding of the electrochemistry of cermet electrode reaction.
In this paper, for the simplification of mathematical process, the complicated network of cermet electrode was assumed to be macroscopically uniform. During the analysis of charge transfer and electrode reaction process of the porous composite electrode, only the overall performance parameters were considered and the apparent effective value were adopted, whereas the subtle structures of the porous electrode were not taken into account. In this way, the complex multiphase network can be characterized and clarified, and the essential characteristic of the whole electrode process can be well exhibited[9]. Due to the large area of actual specific surface,the whole interface electric double layer capacitance is also big, the transient polarization process may be affected markedly by the time constant of the double layer. Thus, it is difficult to characterize the transient polarization process[10]. So, only the steady polarization process was investigated in this work.
2 Electrode structure and equivalent circuit model
A cermet composite electrode has much larger area of actual specific surface than that of a pure metal electrode, which can accelerate the electrode reaction process[11]. Moreover, it has particular mechanics of charge and mass transfer. Porous cermet electrode is composed of metal particles, gas phase pores and electrolyte particles. The metal component, such as Pt and Pd, which is considered to be indispensable, is the basic building block for the electrode[12]. It supplies and transmits electrons for the electrode reaction and allows electronic current to pass through the system. The pores account for about 50% of the total electrode volume. This porous structure facilitates the gas to freely reach the active sites, i.e. tripe phase boundary(TPB), where the electrode reaction takes place[13]. The functions of the electrolyte can be expressed from four aspects as follows. First, it provides mechanical support to the metal particles and suppresses them to agglomerating and clustering. This can maintain the electrode performance for a long time during the reaction process. Second, it extends tripe phase boundary(TPB) from the interface between metal electrode and electrolyte to three-dimensional zone of the whole cermet electrode and so it increases the tripe phase boundary length, which enables the reaction to be more efficient. Third, it brings compatible thermal expansion coefficient close to that of YSZ electrolyte substrate for cermet electrode, so it assures good interface contact between them and stabilizes the structure during the heating-up and operating process[14]. Forth, the continuous distribu- tion of electrolyte phase assists the ionic current to pass through a cermet electrode effectively[15].
Fig.1 schematically shows the structure of a cermet composite electrode. It is known that the electrochemical performance is determined by the constitutional component, microstructure charac- teristics and manufacturing method of the system. Generally, only both electron conduction phase (metal particles) and ionic conducting phase (electrolyte particles) are in continuous state, the cermet electrode just exhibits best performance[16].

Fig.1 Scheme of cermet composite electrode
Fig.2 shows the equivalent circuit model for this system. The porous composite cathode is divided into many thin layers with uniform thickness of dx. The potential of metal phase and electrolyte phase are represented by ![]() and
and![]() , respectively. So, the overpotentialη of the interface between metal phase and electrode phase is given by[9, 17]
, respectively. So, the overpotentialη of the interface between metal phase and electrode phase is given by[9, 17]
![]()
![]() (1)
(1)
where ![]() and
and ![]() are the equilibrium potentials without current for the metal phase and electrolyte phase, respectively;
are the equilibrium potentials without current for the metal phase and electrolyte phase, respectively; ![]() and
and ![]() are potentials of metal phase and electrolyte phase, respectively;
are potentials of metal phase and electrolyte phase, respectively;![]() is a potential constant related to the value of
is a potential constant related to the value of ![]() .
.
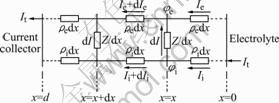
Fig.2 Equivalent circuit model schematic of cermet composite electrode
The Ie and Ii denote the current via the metal phase and electrolyte phase along the x axis, respectively. The ρedx and ρidx represent the ohmic resistance of the metal conductor and electrolyte conductor in each thin layer, wherein ![]() and
and![]() correspond to the effective resistivity. So, the equivalent resistance of the electrochemical reaction at the metal/electrolyte interface in each thin layer (Rr) can be simulated by Rr=Z/dx, wherein Z is the equivalent volume specific resistance. After the overall current(It) enters the electrolyte ionic conductor from x =0, more and more ionic current (Ii) is transformed into electron current(Ie) through the electrochemical reaction at the interface between electrolyte ionic conductor and metal electron conductor. Until x=d, the overall current becomes electron current (Ie) of the metal phase. Obviously, within each dx thin layer, there is
correspond to the effective resistivity. So, the equivalent resistance of the electrochemical reaction at the metal/electrolyte interface in each thin layer (Rr) can be simulated by Rr=Z/dx, wherein Z is the equivalent volume specific resistance. After the overall current(It) enters the electrolyte ionic conductor from x =0, more and more ionic current (Ii) is transformed into electron current(Ie) through the electrochemical reaction at the interface between electrolyte ionic conductor and metal electron conductor. Until x=d, the overall current becomes electron current (Ie) of the metal phase. Obviously, within each dx thin layer, there is
Ie+Ii=It (2)
3 Steady polarization process modeling
The polarization process modelling was based on the following assumptions:
1) Steady polarization state condition.
2) Pressure and temperature are uniform through- out the electrode.
3) One-dimensional model as a function of the x coordinate (Fig.1).
4) Both the conducting phases are considered to be continuous and homogeneous, having a resistivity independent of the x coordinate.
For the cermet electrode, the polarization arises from three aspects, including electrochemical polarization, ohmic polarization and concentration polarization. In order to simplify the mathematic model, the mass transfer in the macropores is neglected. In addition, the concentration polarization is not considered since the gas concentration is assumed to be uniform throughout the electrode[18]. The transfer of charges between the electronic and the ionic conductor can be described by the polarization equation. Based on the Butler-Volmer form regardless of concentration polarization, the polarization equation is written as[19]
![]() (3)
(3)
where i0 is exchange current density; S′is active area per unit volume; η is overpotential of dx thin layer; R is gas constant; T is temperature of electrode.
Introducing parameter b and assuming that α= β=0.5:
![]() (4)
(4)
Then the Eqn.(3) is simplified as
![]()
![]() (5)
(5)
The local current density resulted from the electrochemical reaction is
![]() (6)
(6)
And for porous cermet composite electrode, the conductance of the metal phase is much better than that of the electrolyte phase, that is
ρe<<ρi
So it is considered that the potential of interconnecting metal phase remains constant ![]() . Thus one can get
. Thus one can get
![]() (7)
(7)
Based on Ohm’s law:
![]() (8)
(8)
where the minus means that the current in the electrolyte ionic phase decreases with the increase of x coordinate, one can get
![]() (9)
(9)
Applying differential computing on the above equation, it becomes
![]() (10)
(10)
Substituting Eqn.(6) into Eqn.(10), I* can be expressed as
![]()
![]() (11)
(11)
Combining Eqns.(5) and (11), one can get
![]() (12)
(12)
Eqn.(12) is the essential differential equation of the cathode polarization in cermet composite electrode, without considering the resistance of metal phase and concentration difference polarization. The solution depends on the boundary condition.
![]() (13)
(13)
So
![]() (14)
(14)
![]() (15)
(15)
When x→d, one getsη=0 and d![]() /dx=0.
/dx=0.
Calculating the integral of Eqn.(15), then
![]()
![]() (16)
(16)
![]()
![]() (17)
(17)
wherein the minus was selected since d![]() /dx is always negative. So at x=0,
/dx is always negative. So at x=0,
![]() (18)
(18)
Based on it, one can get
![]()
![]() (19)
(19)
![]()
![]() (20)
(20)
So the cathode polarization formula in cermet composite electrode can be rewritten as
![]() (21)
(21)
Performing integration on Eqn.(17), the following can be obtained:
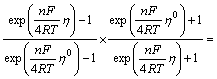
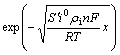 (22)
(22)
4 Discussion
When the electrochemical polarization is small, Eqn.(21) can be rewritten as
![]() (23)
(23)
When electrochemical polarization is large enough, Eqn.(21) can be simplified as
![]() (24)
(24)
Eqn.(24) can be rewritten as
![]() (25)
(25)
In Eqn.(22) defining that
![]() (26)
(26)
L is called as the characteristic thickness of cermet electrode reaction.
Then Eqn.(22) can be rewritten as
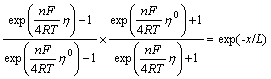 (27)
(27)
It is deduced from Eqn.(27) that ![]() trends to approachη0 when x is large enough.
trends to approachη0 when x is large enough.
According to the above discussion, the η─x/L curve and η0─It curve are drawn in Figs.3 and 4, respectively.
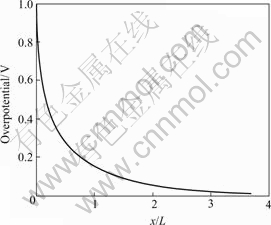
Fig.3 Overpotential distribution curve with x/L
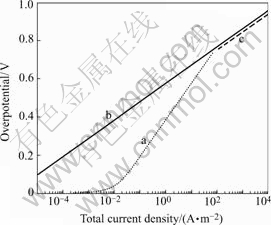
Fig.4 η 0─It polarization curve of cermet electrode
In Fig.3, it can be seen that at the beginning, the overpotential ![]() decreases sharply with the increase of x. However, after x is over 3L, the overpotentialη does not change very much along with the increase of x. For enough thick cermet electrode (the electrode thickness >3.5 L), the overpotential
decreases sharply with the increase of x. However, after x is over 3L, the overpotentialη does not change very much along with the increase of x. For enough thick cermet electrode (the electrode thickness >3.5 L), the overpotential![]() is in close pro- ximity to zero. This suggests that the potential evolutes towards an equilibrium status. If the cermet electrode is too thin, the electrode activity and reaction area become inadequate for the reaction. However, if the cermet electrode is too thick, the excessive portions of cermet which increases the manufacturing cost can not improve the electrode polarization performance. So there should be an optimal range for the thickness of cermet electrode which is approximately between 3.5L and 4.5L.
is in close pro- ximity to zero. This suggests that the potential evolutes towards an equilibrium status. If the cermet electrode is too thin, the electrode activity and reaction area become inadequate for the reaction. However, if the cermet electrode is too thick, the excessive portions of cermet which increases the manufacturing cost can not improve the electrode polarization performance. So there should be an optimal range for the thickness of cermet electrode which is approximately between 3.5L and 4.5L.
Fig.4 depicts the polarization curve of the cermet composite electrode (line a) and a pure noble plane electrode (line b), respectively. It indicates that in the low current density range, the polarization of cermet composite electrode is much smaller than that of the plane electrode. Meanwhile, in the middle current density range, the polarization of cermet composite electrode increases significantly with the increase of It. It is still smaller than that of the plane electrode. When it runs into the high current density range, the polarization of cermet composite electrode eventually keeps increasing to approach that of the plane electrode. What’s more, the slope for line a is about double of that for line b.This is because the effective reaction thickness of cermet electrode decreases sharply with the increase of polarization. From evolution trend of line a, the polarization of cermet composite electrode may surpass that of the plane electrode. In fact, it is impossible in actual cases. When the effective reaction thickness of the cermet electrode approximates to the diameter of microspores in the cermet electrode, the equation deduced in this paper is not valid any more. So in higher polarization range, actual polarization curve of pure noble plane electrode is curve c infinitely approaching polarization line b of the pure noble metal electrode.
5 Conclusions
A continuum model for the polarization resistance and cermet composite electrode reaction has been developed. The electrochemistry polarization behaviors have been discussed in the whole polarization range. The general relationship between the overpotential of the cermet electrode and its thickness is described. Theη0─It curve has been compared with that of a pure noble metal electrode. The results obtained in this paper may enhance the understanding of the electrochemistry related to cermet electrode. Further work is planned to identify the key parameters and give insight into the mechanism of electrode reaction.
List of symbols
b Constant;
d Electrode thickness, m;
F Faraday constant, C/mol;
Ie Axial current of metal phase, A/m2;
Ii Axial current of electrolyte phase, A/m2;
![]() Exchange current density, A/m2;
Exchange current density, A/m2;
![]() Transfer current density per unit of active area, A/m2;
Transfer current density per unit of active area, A/m2;
I t Overall axial current density, A/m2;
L Characteristic thickness of cermet electrode reaction, m;
n Electrons transfered per reacting molecule;
R Gas constant, J/(mol·K) ;
![]() Active area per unit volume, m2/m3;
Active area per unit volume, m2/m3;
T Temperature, K;
x Spatial coordinate along electrode thickness, m;
α, β Charge transfer coefficient;
![]() Overpotential, V;
Overpotential, V;
![]() Overall electrode overpotential, V ;
Overall electrode overpotential, V ;
![]() Equilibrium potential of metal phase, V;
Equilibrium potential of metal phase, V;
![]() Equilibrium potential of electrolyte phase, V;
Equilibrium potential of electrolyte phase, V;
![]() Potential of metal phase, V;
Potential of metal phase, V;
![]() Potential of electrolyte phase, V;
Potential of electrolyte phase, V;
![]() Potential constant. , V;
Potential constant. , V;
![]() Resistivity of electrolyte phase, Ω·m;
Resistivity of electrolyte phase, Ω·m;
![]() Resistivity of metal phase, Ω·m.
Resistivity of metal phase, Ω·m.
References
[1] LEE J Y, Park C O, Beak H D, Hwang H S. Medium-temperature performance of cermet electrode containing Ag and 3Bi2O3·WO3[J]. Sensors and Actuators B, 1995, 28: 211-215.
[2] Sasaki K, Tamura J, Dokiya M. Pt-cermet cathode for reduced temperature SOFCs[J]. Solid State Ionics, 2001, 144: 223-232.
[3] Choi J H, Jang J H, Oh S M. Microstructure and cathodic performance of La0.9Sr0.1MnO3/yttria-stabilized zirconia composite electrodes[J]. Electrochimica Acta, 2001, 46: 867-874.
[4] Adler S B, Lane J A, Steele B C H. Electrode kinetics of porous mixed conducting oxygen electrodes[J]. J Electrochem Soc, 1996, 143: 3554-3564.
[5] Matsuzaki Y, Yasuda I. Electrochemical properties of reduced-temperature SOFCs with mixed ionic-electronic conductors in electrodes and/or interlayers[J]. Solid State Ionics, 2002, 152-153: 463-468.
[6] Sasaki K, Tamura J, Hosoda H, YASUMOTO K, DOKIYA M. Pt perovskite cermet cathode for reduced temperature SOFCs[J]. J Solid State Ionics, 2002, 148: 551-555.
[7] WANG T, Novak R F, Soltis R E. A study of facts that influence zirconia/platinum interfacial impendence using equivalent circuit analysis[J]. Sensors and Actuators B, 2001, 77: 132-138.
[8] Stancovski V, Sridhar S, Pal U B. Thermodynamic stability and interfacial impedance of solid electrolyte cells with noble metal electrodes[J]. J Electroceramics, 1999, 3: 279-299.
[9] ZHA Quan-xing. Introduction to Kinetics of Electrode Process[M]. Beijing: Science Press, 2002.
[10] Athanasiou C, Karagianakis G, Zisekas S, Stoukides m. Electrode polarization at the O2(g), Pd/YSZ interface[J]. Solid State Ionics, 2001, 136-137: 873-877.
[11] Mogensen m, Skaarup s. Kinetic and geometric aspects of solid oxide fuel cell electrode[J]. Solid State Ionics, 1996, 86-88: 1151-1160.0
[12] Sasaki k, Tamura j, Dokiya m. Noble metal alloy–ZrO2 cermet cathode for reduced temperature SOFCS[J]. Solid State Ionics, 2001, 144: 233-240.
[13] Costamagna p, Panizza m, Cerisola g, Barbucci a. Effect of composition on the performance of cermet electrode[J]. J Electrochemical Acta, 2002, 47: 1079-1089.
[14] LIU Mei-lin, WU Zhong-lin. Significance of interfaces in solid state cells with porous electrode of mixed ionic electronic conductors[J]. Solid State Ionics, 1998, 107: 105-110.
[15] costamagna p, costa p, Arato e. Some more consideration on the optimization of cermet solid oxide fuel cell[J]. J Electrochemical Acta, 1998, 43(8): 967-972.
[16] Costamagna p, costa p, Antonucci v. Micro-modelling of solid oxide fuel cell electrodes[J]. Electrochemica Acta, 1998, 43(3-4): 375-393.
[17] sunde s. Monte carlo simulations of polarization resistance of composite for solid oxide fuel cells[J]. J Electrochem Soc, 1996, 143(6): 1930-1939.
[18] Kenjo t, Osawa s, Fujikawa k. High temperature air cathode containing ion conductive oxides[J]. J Electrochem Soc, 1991, 138(2): 349-355.
[19] WU Hao-qing, LI Yong-fang. Electrochemical Kinetics[M]. Beijing: High Education Press, 1998.
Corresponding author: XIAO Jian-zhong; Tel: +86-27-87542800; Fax: +86-27-87544307; E-mail: jzxiao@126.com
(Edited by LI Xiang-qun)


