
Synthesis and characterization of multidoped lithium manganese oxide spinel LiCo0.02La0.01Mn1.97O3.98Cl0.02
ZHANG Na(张 娜), TANG Zhi-yuan(唐致远), HUANG Qing-hua(黄庆华), LU Xing-he(卢星河)
School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China
Received 8 May 2005; accepted 24 August 2005
Abstract:
Multidoped spinel LiCo0.02La0.01Mn1.97O3.98Cl0.02 was synthesized by solid-state method. The structure and electrochemical performance were characterized by XRD, ESEM, particle size distribution analysis, specific surface area testing, galvanostatic cycling and electrochemical impedance spectroscopy. The XRD analysis shows that the sample exhibits pure spinel phase. The substitution of Co, La for Mn and Cl for O in the LiMn2O4 stabilizes the structural integrity of the spinel host, which in turn increases the electrochemical cycleability. The electrochemical experiments confirm that the capacity of the LiCo0.02La0.01Mn1.97O3.98Cl0.02 electrode maintains 90.6% of the initial capacity at 180th cycle.
Key words:
lithium ion batteries; LiMn2O4; lithium manganese oxide spinel; multidoped; electrochemical properties;
1 Introduction
The cathode material plays an important role in the performance of lithium ion batteries. Lithium transition metal compounds with layered and spinel structure are favourites among cathode materials for lithium rechargeable batteries. In this group of materials with high energy capacity, lithium-manganese spinel LiMn2O4 is the most promising one because it is cheaper and less toxic[1-3].
It is well known that manganese ions in LiMn2O4 are in two oxidation states. The increasing Mn3+ content during the intercalation process brings Jahn-Teller distortion. The asymmetric changing of cell parameters (ratio of c to a changing from 10% to 16%) is followed by destruction of spinel structure. Therefore, most research to stabilize cubic spinel structure in recent years has been directed toward the preparation of nonstoichiometric Li-Mn spinels, Mn-substituted stoichiometric LiMn2O4 by low valence cations (<4) [4-8], O-substituted spinel by anoions F and S[9], and surface passivation treatment of LiMn2O4[10].
Spinel LiMn2O4 doped with single metal ions either offers a high capacity similar to the practical capacity obtained from undoped spinel cathode[11], but with limited cycleability upon cycling, or offers relatively stable cycle-life but low initial capacity[12]. In this work, our intention is to synthesize a multidoped spinel material that exhibits the beneficial features of both single-doped spinels[13]. For this purpose, we synthesized a multidoped spinel LiCo0.02La0.01Mn1.97- O3.98Cl0.02 and discussed the productions by substitution of Co, La and Cl for Mn and O, respectively, in both crystal structure and electrochemical properties.
2 Experimental
Both of the samples, LiMn2O4 and LiCo0.02La0.01- Mn1.97O3.98Cl0.02, were synthesized by solid-state reaction of stoichiometric amounts of LiOH·H2O, Mn3O4, Co3O4, La2O3 and LiCl. Well ground of the starting mixtures were preheated at 470 ℃ for 10 h, then ground and calcined at 750 ℃ for 36 h under air flow.
Powder X-ray diffraction(XRD) analyses were carried out on a D/max-2500 XRD diffractometer (Japan) with Cu Ka radiation at 40 kV and 100 mA. Rietveld refinement was then performed on the XRD data to obtain the lattice constants. The scanning electron microscope(SEM) photos were gained on XL 30 ESEM surroundings scanning electron microscope(PHILIPS, Netherlands). The particle distribution for each as-prepared compound were analyzed with MASTERSIZER 2000 laser particle size analyzer (Marlwin Apparatus Company, England). The specific surface area was inspected on CHEMBET300 specific surface area analyzer (QUANTA CHROM, USA).
Electrochemical experiments were performed using coin-type cells. The cathode was prepared by spreading the mixture of 85% active material, 10% acetylene black as conductive additives and 5% polytetrafluoroethylene as binder on to aluminum foil. The prepared electrode was dried in vacuum oven at 120 ℃for 12 h. The cell was assembled in an argon-filled. The cell consisted of a cathode and lithium metal anode separated by American Celgard2300 membrane, using 1 mol/L LiPF6 in a mixture of EC and DMC in mass ratio of 1∶1 as electrolyte. The charge and discharge cycles were carried out with a LandCT2001 battery program-control test system at current of 0.2C rate over a potential range between 3.0 and 4.3 V.
The AC impedance measurement was performed using CHI660B electrochemical interface. Before AC impedance measurements, the cell was pre-cycled between 3.0 and 4.3 V for five cycles to establish and stabilize the solid electrolyte interface(SEI) between the electrolyte and electrodes. The cell was then potentiostatically conditioned to a potential and equilibrated for 2 h, then, AC impedance spectra were obtained by applying a sine wave of 5 mV amplitude over the frequency range of 100 kHz to 10 mHz.
3 Results and discussion
3.1 Structure and physical characterization
The structure of the as-prepared powders of undoped spinel and (Co, La, Cl)-doped spinel were characterized by XRD. Their XRD patterns are shown in Fig.1. Both of the samples were identified as a single phase of cubic spinel with a space group Fd3m. The cubic lattice parameters were listed in Table 1. As compared with that of LiMn2O4, the doped spinel indicates smaller lattice parameters with the doping of Co, La, Cl ions in the spinel structure.
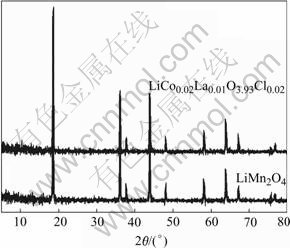
Fig.1 XRD patterns of LiMn2O4 and LiCo0.02La0.01Mn1.97- O3.98Cl0.02 powders
Table 1 Lattice parameters of as-prepared spinels

The ESEM of the synthesized LiMn2O4 and LiCo0.02La0.01Mn1.97O3.98Cl0.02 powders were observed by scanning electron microscopy, the results are shown in Fig.2. Both LiMn2O4 and LiCo0.02La0.01Mn1.97O3.98Cl0.02 exhibit similar particle shape. Despite the sample process condition, the spinel powder shows a relatively smooth surface appearance and large particle size by multi-doping with Co, La and Cl ions.

Fig.2 ESEM images of LiMn2O4(a) and LiCo0.02La0.01Mn1.97- O3.98Cl0.02(b)
The particle sizes of LiMn2O4 and LiCo0.02La0.01- Mn1.97O3.98Cl0.02 powders were also measured. The particle properties of LiMn2O4 and LiCo0.02La0.01Mn1.97- O3.98Cl0.02 are listed in Table 2. It can be seen that the mean particle size of the two samples are 4.034 and 5.628 μm. Compared with the substituted sample, LiMn2O4 shows a slightly larger particle size value, which is consistent with the ESEM results. The corresponding data of specific area of the two samples are also summarized in Table 2, which shows that the specific surface area of LiCo0.02La0.01Mn1.97O3.98Cl0.02 is lower than the one for LiMn2O4 due to the smooth surface and the larger particle size.
Fig.3 shows the first charge-discharge curves of LiMn2O4 and LiCo0.02La0.01Mn1.97O3.98Cl0.02 cathodes. It can be obviously seen that the charge-discharge curves of the two samples have two voltage plateaus at approximately 4.05 and 4.15 V, which is a remarkable characteristic of well-defined LiMn2O4 spinel. From charge-discharge curves, it is found that lithium ion is reversibly lithiated/delithiated through LiMn2O4 spinel framework. The initial capacity of LiMn2O4 obtained in this work is 125.9 mA·h/g (theoretical capacity of LiMn2O4 is 148 mA·h/g). By doping with Co, La and Cl, the initial capacity decreased to 119.9 mA·h/g. This is due to Co, La substations for Mn to decrease of Mn3+ amount in the substituted spinel phase since during the intercalation/deintercalation of Li+ in the LiMn2O4 matrix only the amount of Mn3+ contributes to the charge/discharge capacity.
Table 2 Particle properties of LiMn2O4 and LiCo0.02La0.01Mn1.97O3.98Cl0.02

Fig.4 shows the comparison of the cycle performance of LiMn2O4 and LiCo0.02La0.01Mn1.97O3.98Cl0.02 powders. Obviously, the substituted spinel phase is more stable than the LiMn2O4 spinel phase, the effect of dopant incorporation is a subsequent improvement of Li/LiMn2O4 cyclability. The electrode of LiCo0.02La0.01- Mn1.97O3.98Cl0.02 retains 90.6% (108.6 mA·h/g) of the initial capacity after 180 cycles at 0.2C rate. The electrochemical stability has been attributed to the stronger metal-oxygen bonding in substituted LiCo0.02La0.01Mn1.97O3.98Cl0.02 spinel phase and the substitution of Cl for O is very effective in hindering the formation of the tetragonal Li2Mn2O4 in the low voltage range[14].
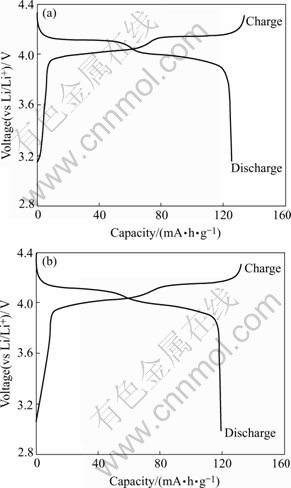
Fig.3 First charge-discharge curves of Li/LiMn2O4(a) and Li/LiCo0.02La0.01Mn1.97O3.98Cl0.02(b) cells
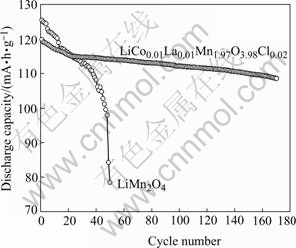
Fig.4 Cycle performance of Li/LiMn2O4 and Li/LiCo0.02La0.01- Mn1.97O3.98Cl0.02 cells
Electrochemical impedance spectroscopy aids understanding the electrode kinetics by analyzing the variation in impedances associated with different processes during lithium intercalation/deintercalation reaction[15]. Fig.5 shows Nyquist plots of LiMn2O4 and LiCo0.02La0.01Mn1.97O3.98Cl0.02 electrode at different charging voltages. All the impedance spectra show a high-frequency semicircle with a maximum at a frequency of a few kilohertz, a medium-frequency semicircle, which is evident at highly charged state and low frequencies, the typical behavior of a diffusion process inside the electrode materials. We ascribed the high-frequency semicircle to the charge of the external surface area of the lithium manganese oxide composite electrodes and the related resistance to both interparticle electronic contact and ionic migration through the passivation layer. The medium-frequency semicircle resistance is assigned to the Faradic charge process of lithium manganese oxide and the interfacial capacitance. In the charge state the Li/LiCo0.02La0.01Mn1.97O3.98Cl0.02 system generates a lower cell impedance than that of Li/LiMn2O4 system as a consequence of good thermodynamics and reasonable reaction kinetics. The impedance patterns further verify that the LiCo0.02La0.01- Mn1.97O3.98Cl0.02 system has excellent electrochemical performance.
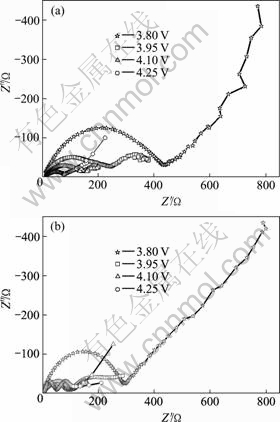
Fig.5 Nyquist plots recorded for Li/LiMn2O4(a) and Li/ LiCo0.02La0.01Mn1.97O3.98Cl0.02(b) system at different charging voltages
4 Conclusions
A new doped spinel LiCo0.02La0.01Mn1.97O3.98Cl0.02 was synthesized by a solid-state method. XRD result reveals that the structure of the substituted compound retains cubic spinel phase. The powder has similar morphology and particle size distribution with undoped LiMn2O4, but larger particle size and lower specific area. The good electrochemical performance of the Li/LiCo0.02La0.01Mn1.97O3.98Cl0.02 system is confirmed by the charge-discharge curve and the electrochemical impedance spectra. The LiCo0.02La0.01Mn1.97O3.98Cl0.02 electrode shows excellent cycleability, which retains 90.6% (108.6 mA·h/g) of the initial capacity after 180 cycles at 0.2C rate.
References
[1] LIU R S, SHEN C H. Structural and electrochemical study of cobalt doped LiMn2O4 spinels[J].Solid State Ionics, 2003,157 (1): 95-100.
[2] Kim Y S, Kanoh H, Horotsu T, OOI K. Chemical bonding of ion-exchange type sites in spinel-type manganese oxides Li1.33Mn1.67O4[J]. Mater Res Bull, 2002, 37(2): 391-396.
[3] Chebiam R V, Kannan A M, Prado F, MANTHIRAM A. Comparison of the chemical stability of the high energy density cathodes of lithium-ion batteries[J]. Electrochem Commun, 2001, 3(11): 624-627.
[4] Thirunakarana R, Kimb K T, Kangb Y M, YONG-LEE J. Cr3+ modified LiMn2O4 spinel intercalation cathodes through oxalic acid assisted sol–gel method for lithium rechargeable batteries[J]. Mater Res Bull, 2005, 40(1): 177-186.
[5] Sun Y K, Jeon Y S, Leeb H J. Overcoming Jahn-Teller distortion for spinel Mn phase[J]. Electrochem Solid-State Lett, 2000, 3(1): 7-9.
[6] Julien C, Mangani I R, Selladurai S, MASSOT A. Synthesis, structure and electrochemistry of LiMn2-yCry/2Cuy/2O4 (0.0≤y≤0.5) prepared by wet chemistry[J].Solid State Sci, 2002, 4(8): 1031- 1038.
[7] ZHAO Ming-shu, SONG Xiao-ping. Preparation and characterization of LiMn1.5Me0.5O4 (Me= Ti, Fe, Ni, Zn) for lithium-ion battery cathode materials[J]. Trans Nonferrous Met Soc China, 2004, 14(4): 811-816.
[8] ZHAO Ming-shu, ZHAI Yu-chun, TIAN Yan-wen. Preparation and characterization of LiMn1.75Me0.25O4 (Me=Ti, Fe, Ni) for lithium-ion battery cathode material[J]. The Chinese Journal of Nonferrous Metals, 2000, 12(4): 733-738.(in Chinese)
[9] SUB Y K, OH I H. Cycling behavior of oxysulfide spinel LiCr0.19Mn1.81O3.98S0.02 cathode material which shows no capacity loss in the 3-V region[J]. J Power Source, 2001, 94(1): 132-136.
[10] Saitoh M, Yoshida S, Yamane H, SANO M, FUJITA M, KIFUNE K, KUBOTA Y. Capacity fading of the acid-treated lithium manganese oxides in high-temperature storage[J]. J Power Sources, 2003, 122(2): 162- 168.
[11] ZHONG Q, Bonaldarpour A, Zhang M, GAO Y, DAHN J R. Synthesis and Electrochemistry of LiNixMn2–xO4[J]. J Electrochem Soc, 1997, 144(1): 205-213.
[12] Ein-Eli Y, Howard W F. LiCuCuMnO4: 5 V Cathode Materials[J]. J Electrochem Soc, 1997, 144(8): L205- L207.
[13] Hwang B J, Santhanam R, Hu S G. Synthesis and characterization of multidoped lithium manganese oxide spinel, Li1.02Co0.1Ni0.1Mn1.8O4, for rechargeable lithium batteries[J]. J Power Source, 2002, 108(2): 250-255.
[14] Song D, Ikuta H, Uchida T, WAKIHARA M. The spinel phases LiAlyMn2-yO4 (y=0, 1/12, 1/9, 1/6, 1/3) and Li(Al,M)1/6Mn11/6O4 (M=Cr, Co) as the cathode for rechargeable lithium batteries[J]. Solid State Ionics, 1999, 117(2): 151-156.
[15] Arbizzani C, Balducci A, Mastragostino M, ROSSI M, SOAVI F. Characterization and electrochemical performance of Li-rich manganese oxide spinel/poly(3,4- ethylenedioxythiophene) as the positive electrode for lithium-ion batteries[J]. J Electroanalytical Chemistry, 2003, 553(1): 125-133.
Foundation item: Project(20273047) supported by the National Natural Science Foundation of China
Corresponding author: TANG Zhi-yuan; Tel: +86-22-27892832; Fax: +86-22-27401797; E-mail: zhangna0059@163.com
Abstract: Multidoped spinel LiCo0.02La0.01Mn1.97O3.98Cl0.02 was synthesized by solid-state method. The structure and electrochemical performance were characterized by XRD, ESEM, particle size distribution analysis, specific surface area testing, galvanostatic cycling and electrochemical impedance spectroscopy. The XRD analysis shows that the sample exhibits pure spinel phase. The substitution of Co, La for Mn and Cl for O in the LiMn2O4 stabilizes the structural integrity of the spinel host, which in turn increases the electrochemical cycleability. The electrochemical experiments confirm that the capacity of the LiCo0.02La0.01Mn1.97O3.98Cl0.02 electrode maintains 90.6% of the initial capacity at 180th cycle.


