文章编号:1004-0609(2010)04-712-06
电沉积制备(Ni-W-P)-TiO2纳米复合电极的催化析氢性能
李爱昌,骆鹏飞,刘 瑛
(廊坊师范学院 化学与材料科学学院, 廊坊 065000)
摘 要:
用恒电流复合电沉积方法制备(Ni-W-P)-TiO2复合电极,讨论TiO2悬浮量和电沉积时间对电极催化析氢性能的影响。采用SEM和XRD对电极的表面形貌和晶体结构进行分析,以稳态极化曲线对电极的催化析氢特性进行评价,并推测其反应机理。结果表明:(Ni-W-P)-TiO2电极是纳米TiO2粒子相和纳米晶Ni-W-P固溶体相构成的复合电极,具有较高的催化析氢活性;在25 ℃、0.5 mol/L H2SO4介质中(Ni-W-P)-TiO2电极的表观交换电流密度是Ni-W-P合金电极的2.7倍,是Ni电极的53倍;当电流密度为100 mA/cm2时,该电极电势相对于Ni-W-P电极正移了176 mV,相对于Ni电极正移了 581 mV;(Ni-W-P)-TiO2复合电极催化活性的提高主要源于反应机理的改变,复合电极表面的TiO2纳米粒子与Ni-W-P合金具有明显的电子协同效应。
关键词:
(Ni-W-P)-TiO2电极;Ni-W-P电极;Ni电极;复合电沉积;电催化;析氢;
中图分类号:TG146.1; O646 文献标志码:A
Hydrogen evolution properties of (Ni-W-P)-TiO2 composite coating as electrode materials prepared by electrolytic co-deposition
LI Ai -chang, LUO Peng-fei, LIU Ying
(Faculty of Chemistry and Material Science, Langfang Teachers College, Langfang 065000, China)
Abstract: (Ni-W-P)-TiO2 composite coating used as electrode materials was prepared by electrolytic co-deposition at constant current. The effects of concentration of TiO2 particles suspended in the plating bath and depositing time on the catalytic activity of the electrode for the hydrogen evolution reaction (HER) were discussed. The surface morphology and phase structure of (Ni-W-P)-TiO2 coating were observed by SEM and XRD. The catalytic activity of the HER was evaluated on the basis of electrochemical steady-state polarization curves. And the reaction mechanism was presumed. The results show that the (Ni-W-P)-TiO2 electrode consists of TiO2 crystalline and Ni-W-P nano crystalline in solid solution. The composite electrode is catalytically more active than Ni-W-P or Ni alloy electrode. The apparent exchange current density of the composite electrode in 0.5 mol/L H2SO4 solution is 2.7 times that of the Ni-W-P electrode, and 53 times that of Ni electrode at 25 ℃. The polarization overpotential for hydrogen evolution of the (Ni-W-P)-TiO2 electrode is 176 mV lower than that of Ni-W-P electrode and is 581 mV lower than that of Ni electrode at the current density of 100 mA/cm2. The increased catalytic activity for the HER of the (Ni-W-P)-TiO2 composite electrode is mainly due to the change in the reaction mechanism caused by the synergistic electronic effect of the Ni-W-P alloy and the TiO2 nano particles on the electrode surface.
Key words: (Ni-W-P)-TiO2 electrode; Ni-W-P electrode; Ni electrode; composite electroplating; electrocatalysis; hydrogen evolution
氢能作为无污染的生态清洁能源,受到各国科技工作者的高度关注。电解水制氢是实现工业化制氢的重要手段,为降低能耗,最有效的方法是降低阴极析氢超电势。因此,开发新型廉价高效的析氢材料具有十分重要的意义[1]。不少文献中已报道过关于将过渡金属合金[2-3]、NiS[4]、RaneyNi[5]以及各种复合镀层[6-9] 作为电解析氢反应催化层的研究。结果表明:Ni-W-P合金[10-11]与Ni-Mo合金的析氢性能相当,且优于Ni-S、RaneyNi、Ni-Zn、Ni-Fe和Ni-W等金属材料的析氢性能,但其在催化活性和抗杂质干扰方面仍有待于提 高[12]。GIERLOTKA等[7]曾报道在Ni-P合金中引入微米级TiO2粒子形成(Ni-P)-TiO2复合镀层,改善了电极的催化活性;蔡乃才等[8]和邹勇进等[9]分别将微米级RuO2(2 μm)和TiO2(3 μm)引入Ni-Mo合金和Ni-W合金中形成复合电催化剂层,使合金镀层的催化性能得到不同程度的改善。此外,人们较多地关注了电极在碱性介质中的析氢行为,很少研究电极在酸性介质中的析氢特性[13-15],尽管一些电极具有优异的抗腐蚀性能。本文作者采用复合电沉积技术,将P-25纳米TiO2微粒掺入到Ni-W-P合金镀层中,制备(Ni-W-P)-TiO2纳米镀层电极,并对其在酸性介质中的电催化析氢性能进行研究。
1 实验
实验在超级恒温水浴控制下的恒温镀槽中进行,采用恒电流沉积,用调速搅拌器搅拌,使TiO2微粒均匀悬浮于400 mL镀液中。将不锈钢板作阳极,紫铜片(1 cm×2 cm)作阴极,非工作面用环氧树脂封固。所用TiO2是P-25纳米粉(德国Degussa公司生产,80%(质量分数)锐钛矿相,20%金红石相)。镀前悬浮液超声处理30 min。镀液组成[10]及操作条件如表1所示。
表1 电沉积(Ni-W-P)-TiO2电极的镀液组成及实验条件
Table 1 Bath compositions and experimental conditions of (Ni-W-P)-TiO2 electrode

电极的表面形貌用FEI Quanta 200 FEG场发射扫描电镜观察,并以Oxford 7426型能谱仪测定电极表面的组成。用日本理学2500型X射线衍射仪(Cu Kα)以3?掠角测定镀层的结构。
利用辰华CHI660C电化学工作站进行电化学测试。测试溶液为0.5 mol/L H2SO4,阴极极化曲线的扫描速率为2 mV/s。辅助电极为大面积铂网,参比电极为饱和甘汞电极(SCE)。测定前向电解液中通氮气20 min,以除去溶液中可能存在的氧气。溶液的欧姆电势降用电化学工作站自带软件补偿。
2 结果与讨论
2.1.1 镀液中TiO2悬浮量的影响
(Ni-W-P)-TiO2电极的沉积条件如表1所列。在表1条件下,控制电镀时间为40 min,使镀液中TiO2的悬浮量分别为2.5、5.0、7.5、10.0、12.5、15.0 g/L,制备6种电极分别记为电极a~f。图1所示为各电极在25 ℃、0.5 mol/L H2SO4溶液中的极化曲线。由图1可知,随着镀液中TiO2悬浮量的增加,电极的析氢催化活性先增大而后减小,悬浮量为12.5 g/L时所制电极e的催化活性最佳。这是复合电极的催化活性随电极中TiO2含量增大而增加所致[16]。随着镀液中TiO2悬浮量的增加,单位时间内通过搅拌等作用被输送到阴极表面的TiO2微粒数量增多,微粒进入镀层的几率也增大,这是TiO2在镀层中的含量随镀液中微粒悬浮量增大而增大的原因;由于TiO2纳米微粒容易团聚,
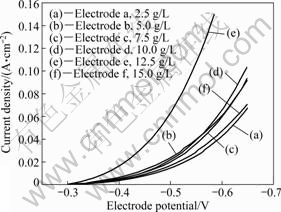
图1 TiO2悬浮量对电极催化活性的影响
Fig.1 Effects of concentration of TiO2 particles suspended in plating bath on catalytic activity for hydrogen evolution reaction
当镀液中微粒含量过大时,TiO2微粒聚结为大颗粒,从而有可能使不同粒径的粒子的总浓度减小,进而使镀层中微粒的含量降低。本文作者在沉积(Ni-Mo)-TiO2复合镀层时也发现了这个规律。因此,在制备纳米复合镀层时,克服微粒在高浓度的团聚至关重要。
2.1.2 沉积时间的影响
在表1所列条件下,固定TiO2的悬浮量为12.5 g/L,探讨电沉积时间对电极催化活性的影响(25 ℃、0.5 mol/L H2SO4),结果如图2所示。 由图2可知,电沉积时间对电极催化活性有明显的影响,电沉积40 min所制备电极的析氢活性最高。这一变化规律与电极表面的化学组成和比表面积随电沉积时间变化 有关。

图2 沉积时间对电极催化活性的影响
Fig.2 Effects of depositing time on catalytic activity of electrode for hydrogen evolution reaction
图3(a)所示为在表1所示条件下,(TiO2悬浮量为0,电沉积时间为40 min所制备的Ni-W-P电极的表面形貌;图3(b)所示为在表1所示条件下,TiO2悬浮量为12.5 g/L、电沉积时间为40 min所制的(Ni-W-P)- TiO2电极的SEM像。
对比图3(a)和(b)可知,电极表面均由大小不等的颗粒构成,相对于合金电极的表面粗糙度,复合电极的表面粗糙度增大,这有利于析氢反应的进行。图3(c)所示为(Ni-W-P)-TiO2电极的高倍SEM像。能谱分析表明,大的球形颗粒为Ni-W-P合金,粒径在0.3~0.8 μm范围内。图3(c)中松散、不连续的小虫卵状颗粒为TiO2纳米粒子。TiO2纳米粒子出现一定程度的团聚现象,但大部分颗粒在100~300 nm。EDS分析表明,复合电极中TiO2的含量为3.62%(质量分数)。
图4所示为合金电极和复合电极的XRD谱。由图4可知,复合镀层在2θ=25.42?和48.23?出现锐钛矿TiO2的第1和第2衍射峰,其他3个峰的峰位与Ni-W-P合金镀层的基本相同。这表明TiO2微粒的引入并没有改变Ni-W-P合金镀层的晶相结构。此外,谱图中未出现金红石相TiO2的衍射峰,这可能是其含量低的缘故。

图3 不同镀层电极的SEM像
Fig.3 SEM images of different coating electrodes: (a) Ni-W-P; (b), (c) (Ni-W-P)-TiO2
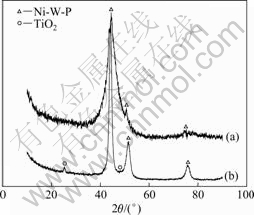
图4 不同镀层电极的XRD谱
Fig.4 XRD patterns of different coating electrodes: (a) Ni-W-P; (b) (Ni-W-P)-TiO2
由图4还可知,Ni-W-P合金中3个强衍射峰位依次为44.34?、50.74?和75.61?,这与Ni(PDF040850)的3个强衍射峰位44.51?、51.85?和76.37?接近。考虑到纯W的3个强峰位依次为40.26?、58.36?和73.38?,P的3个强峰位为37.98?、54.58?和68.36?,在Ni-W-P的XRD谱中这些位置及附近并未出现W和P的衍射峰,说明W和P在合金中并没有独立成相,而是与Ni一起形成了新相。基于上述实验结果,本文作者认为Ni-W-P合金是以Ni为溶剂,W和P为溶质的置换型固溶体合金。Ni-W-P合金的衍射峰位相对于Ni的偏离是由W和P原子取代Ni原子的晶格位置时产生的晶格畸变造成的[17]。分别取Ni-W-P合金电极和(Ni-W-P)-TiO2电极的最强衍射峰,依据Scherrer公 式[17]计算晶粒平均尺寸,结果分别为2.14和7.33 nm。由此可知,(Ni-W-P)-TiO2镀层电极是由纳米TiO2粒子相与平均尺寸为7.33 nm的纳米晶Ni-W-P固溶体合金相构成的复合电极。
2.3.1 Tafel参数
图5所示为Ni电极、Ni-W-P电极和(Ni-W-P)- TiO2复合电极在25 ℃、0.5 mol/L H2SO4中的阴极极化曲线。由图5可知,在电流密度为100 mA/cm2时,Ni的电极电势为-1.124 V,Ni-W-P电极的电势为-0.719 V,(Ni-W-P)-TiO2复合电极的电势为-0.543 V,即在该电流密度下,复合电极的电势相对于Ni-W-P电极正移了176 mV,相对于Ni电极正移了581 mV。

图5 在25 ℃、0.5 mol/L H2SO4中不同电极的阴极极化曲线
Fig.5 Cathodic polarization curves of different coating electrodes in 0.5 mol/L H2SO4 at 25 ℃
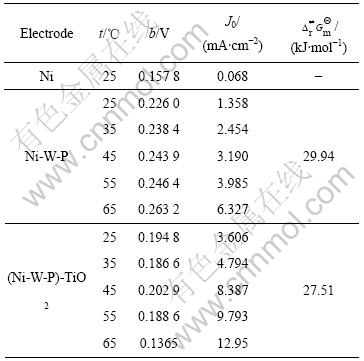
根据Butler-Volmer方程,求得3种电极的析氢动力学参数,如表2所示。由表2可知,25 ℃时(Ni-W-P)-TiO2复合电极的表观交换电流密度是Ni-W-P电极的2.7倍,是Ni电极的约53倍。由以上结果可知,(Ni-W-P)-TiO2电极在H2SO4介质中表现出较高的催化析氢活性。
表2 催化析氢电极的Tafel动力学参数
Table 2 Tafe kinetic parameters of catalytic activity of electrodes for hydrogen evolution
2.3.2 标准表观活化吉布斯自由能
根据Arrhenius定律,电极析氢反应的表观交换电流密度J0(或真实交换电流密度Jr, 0)与表观活化吉布斯自由能![]() 和温度符合以下关系:
和温度符合以下关系:

若以Jr, 0取代式(1)中的J0,则只是常数K不同。以lgJ0对1/T作图,可得到电极催化析氢反应表观活化吉布斯自由能![]() ,结果如图6所示。
,结果如图6所示。
通过计算得到Ni-W-P电极的表观活化吉布斯自由能为29.94 kJ/mol,(Ni-W-P)-TiO2电极的为27.51 kJ/mol。由于纳米TiO2引入到Ni-W-P合金后,电极析氢反应的表观活化吉布斯自由能降低了2.43 kJ/mol。由此可知,虽然(Ni-W-P)-TiO2复合电极催化析氢活性的提高可归因于能量因素和几何因素(比表面积增大,见图3),但能量因素占据主导地位。
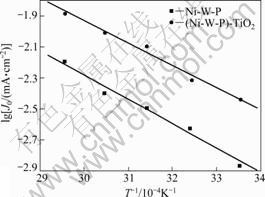
图6 电极的表观电流密度对数与T-1的关系
Fig.6 Relationship between apparent current density and T-1
目前,关于Ni-Mo(W)和Co-Mo(W)电极以及Pt-WO3电极所显示的电催化活性,一般用包含氢原子溢出(spillover)的协同效应来解释[18],Ni-W-P电极的催化机理也应如此,即按下述步骤进行:

在酸性溶液和高超电势条件下,一般按式(4)进行电化学脱附。式中:H(ads)表示H吸附原子,M表示除Ni以外的其它金属原子(对Ni-W-P为WP,对Ni-Mo为Mo等)。溶液中的H+首先在电极表面Ni的活性位上放电形成吸附在Ni上的吸附氢原子Ni-H(ads),而后Ni-H(ads)由Ni向M的活性位上扩散形成M-H(ads)。这就是所谓的氢原子由Ni向其它金属原子“溢出”,最后按式(4)或(5)进行氢的脱附。在高超电势下,H(ads)在Ni表面的覆盖度很大,析氢反应为式(2)(Volumer反应)控制(这可由表2中的b值大于0.12 V得出结论)。正是通过H(ads)的溢出使H(ads)在Ni表面的覆盖度减小,从而加速了催化反应[13]。
(Ni-W-P)-TiO2电极相对于Ni-W-P电极催化活性提高的机制如何呢?GIERLOTKA等[7]针对(Ni-P)-TiO2复合电极在酸性介质中的催化析氢反应提出了如下机理:

即镀层中的TiO2只是作为一种独立成分参与催化反应,而与Ni-P合金没有相互作用。邹勇进等[9]也以此解释了(Ni-W)-TiO2复合电极中固体微粒的析氢作用。本文作者认为这种机制值得商榷。
楮道葆等[19-20]用循环伏安法对纳米TiO2/Ti电极在1 mol/L H2SO4中的催化性能进行了研究,发现在电极电势φ>-1.2 V(vs SCE)范围内未出现氢气的析出。这说明若不考虑Ni-P合金的作用,在实验的电势区间(自稳定电势负扫至-0.7 V)[7],(Ni-P)-TiO2复合电极中的TiO2微粒不可能有析氢作用,即复合电极的催化作用不可能高于Ni-P合金电极,这与作者的实验结果相矛盾。因此,在复合电极中TiO2微粒与金属间一定存在着某种明显的相互作用。
林化新[21]曾用动电位扫描法对铂修饰的锐钛矿TiO2粉末电极(Pt/TiO2)的性能进行了探究,结果发现在电极上存在明显的氢溢流现象;并且随着金属Pt担载量的增加,这种溢流作用明显增强。此外,从氢溢流有关理论进行分析,因为Ti属于过渡金属,其Ti-H吸附键强度与W、Mo的相差不大,属于较强的吸附键,完全可能产生吸附氢自Ni、Pt至TiO2的“溢流”作用。
基于上述考虑,本文作者认为镀层中的TiO2纳米粒子在电催化析氢中的作用与Mo或W相当,即捕获来自于Ni活性位上的吸附氢H(ads)。正是这种效应加速了反应的进行。因此,对于(Ni-W-P)-TiO2复合电极催化析氢反应的机理确定如下:
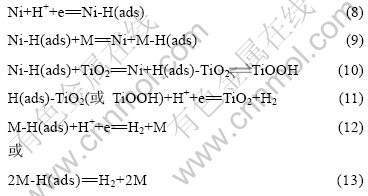
3 结论
1) 采用复合电沉积方法制备(Ni-W-P)-TiO2电极,最佳工艺条件如下:pH值为6.0,温度为30 ℃,电流密度为150 mA/cm2,搅拌速度为380 r/min,TiO2悬浮量为12.5 g/L,电沉积时间为40 min。
2) XRD测试表明,(Ni-W-P)-TiO2镀层电极是由纳米TiO2粒子相和纳米晶Ni-W-P固溶体相构成的复合电极。
3) 采用稳态极化曲线测试,评价了复合电极的催化析氢性能,并与Ni电极和相同条件下制备的Ni-W-P电极进行了比较。结果表明,(Ni-W-P)-TiO2电极具有较高的催化析氢活性,在25 ℃、0.5 mol/L H2SO4中,复合电极的表观交换电流密度是Ni-W-P电极的2.7倍,是Ni电极的53倍。在电流密度为100 mA/cm2时,复合电极的电极电势相对于Ni-W-P电极正移了176 mV,相对于Ni电极正移了581 mV。
4) 复合电极催化活性的提高主要源于反应机理的改变。复合电极中的TiO2纳米粒子作为活性吸附氢的捕获剂参与了氢溢流过程,产生了正电子协同效应,提高了电极催化析氢反应活性。
REFERENCES
[1] Paunovié P, Popovski O, Dimitrov A. Study of structural and electrochemical characteristics of Co-based hypo-hyper d-electrocatalysts for hydrogen evolution[J]. Electrochimica Acta, 2007, 52: 4640-4648.
[2] JAKˇSI?C J M, VOJNOVI?C M V, KRSTAJI?C N V. Kinetic analysis of hydrogen evolution at Ni-Mo alloy electrodes[J]. Electrochim Acta, 2000, 45: 4151-4158.
[3] METIKOˇS-HUKOVI?C M, JUKI?C A. Correlation of electronic structure and catalytic activity of Zn-Ni amorphous alloys for the hydrogen evolution reaction[J]. Electrochim Acta, 2000, 45: 4159-4170.
[4] TSEUGN A C, Antonitam A J, Hibbert D B. Energy conservation in the chlor-alka industry[J]. Chemistry and Industry, 1984, 16: 54-60.
[5] Lonrber Q K, Kohl P. Preparation and use of raney-Ni activated cathodes for large scale hydrogen production[J]. Electrochimica Acta, 1984, 29(11): 1557-1561.
[6] Wu G, Li N, Dai C S, ZHOU D R. Electrochemical preparation and characteristics of Ni-Co-LaNi5 composite coatings as electrode materials for hydrogen evolution[J]. Materials Chemistry and Physics, 2004, 83: 307-314.
[7] Gierlotka D, Rówiński E, Budniok A. Production and properties of electrolytic Ni-P-TiO2 composite layers[J]. Journal of Applied Electrochemistry, 1997, 27: 1349-1354.
[8] 蔡乃才, 桂岳琼, 黄 勤. 高比表面Ni-Mo-RuO2复合催化层析氢电极[J]. 武汉大学学报: 自然科学版, 1999, 45(2): 157-159.
CAI Nai-cai, GUI Yue-qiong, HUANG Qin. High area Ni-Mo-RuO2 composite coating as hydrogen electrode[J]. Journal of Wuhan University: Natural Science Edition, 1999, 45(2): 157-159.
[9] 邹勇进, 肖作安, 费锡明, 任新林. Ni-W-TiO2复合镀工艺及其镀层性能研究[J]. 材料保护, 2004, 37(12): 24-26.
ZOU Yong-jin, XIAO Zuo-an, FEI Xi-ming, REN Xin-lin. Electrochemical preparation and characteristics of Ni-W-TiO2 composite coating[J]. Materials Protection, 2004, 37(12): 24-26.
[10] 李爱昌, 姚素薇, 赵水林, 郭鹤桐. 电沉积镍钨磷合金催化析氢特性研究[J]. 表面技术, 1995, 24(3): 8-10.
LI Ai-chang, YAO Su-wei, ZHAO Shui-lin, GUO He-tong. Study of electrodeposited Ni-W-P as hydrogen electrode[J]. Surface Technology, 1995, 24(3): 8-10.
[11] 张卫国, 刘 洋, 李 贺, 姚素薇. 热处理对电沉积镍钨磷合金催化析氢性能的影响[J]. 催化学报, 2006, 27(11): 967-970.
ZHANG Wei-guo, LIU Yang, LI He, YAO Su-wei. Effect of heat treatment on the electrocatalytic activity of electrodeposited Ni-W-P alloy for hydrogen evolution reaction[J]. Chinese Journal of Catalysis, 2006, 27(11): 967-970.
[12] Divisek J, Schmitz H, Steffen B. Electrocatalyst materials for hydrogen evolution[J]. Electrochimica Acta, 1994, 39(12): 1723-1731.
[13] MARTINEZ S, METIKOˇS-HUKOVI?C M, VALEK L. Electrocatalytic properties of electrodeposited Ni–15Mo cathodes for the HER in acid solutions: Synergistic electronic effect[J]. Journal of Molecular Catalysis A: Chemical, 2006, 245: 114-121.
[14] LU G, EVANS P, ZANGARI G. Electrocatalytic properties of Ni-based alloys towards hydrogen evolution reaction in acid media[J]. J Electrochem Soc, 2003, 150(5): 551-557.
[15] LU G, ZANGARI G. Corrosion resistance of ternary Ni-P based alloys in sulfuric acid solutions[J]. Electrochim Acta, 2002, 47: 2969-2979.
[16] 李爱昌, 傅 丽, 龙运前, 王 芳. 复合电沉积法制备Sn-TiO2纳米薄膜[J]. 材料保护, 2007, 40(4): 32-34.
LI Ai-chang, FU Li, LONG Yun-qian, WANG Fang. Study on the composite electroplating process for Sn-TiO2 nanofilm and its photocatalytic activity[J]. Materials Protection, 2007, 40(4): 32-34.
[17] RADMILOVIC V, GASTEIGER H A, ROSS P N. Structure and chemical composition of a supported Pt-Ru electrocatalyst for methanol oxidation[J]. J Catal, 1995, 154(1): 98-106.
[18] TSEUNG A C C, CHEN K Y. Hydrogen spill-over effect on Pt/WO3 anode catalysts[J].Catalysis Today, 1997, 38: 439-443.
[19] 褚道葆, 沈广霞, 周幸福, 林昌健, 林华水. Ti表面修饰纳米TiO2膜电极的电催化活性[J]. 高等学校化学学报, 2002, 23(4): 678-681.
CHU Dao-bao, SHEN Guang-xia, ZHOU Xing-fu, LIN Chang-jian, LIN Hua-shui. Eletrocatalytic activity of nanocrystalline TiO2 film modified Ti electrode[J]. Chem J Chinese Universities, 2002, 23(4): 678-681.
[20] 顾家山, 褚道葆, 周幸福, 沈广霞. 纳米TiO2膜修饰电极异相电催化还原马来酸[J]. 化学学报, 2003, 61(9): 1405-1409.
GU Jia-shan, CHU Dao-bao, ZHOU Xing-fu, SHEN Guang-xia. Heterogeneous electrocatalytic reduction of maleic acid on nanocrystalline TiO2 film modified Ti electrode[J]. Acta Chimica Sinica, 2003, 61(9): 1405-1409.
[21] 林化新. 用动电位扫描法研究Pt/TiO2上的氢溢流和反溢流[J]. 催化学报, 1991, 12(3): 187-192.
LIN Hua-xin. Study of hydrogen spillover and back spillover on Pt/TiO2 by potential dynamic sweep method[J]. Journal of Catalysis, 1991, 12(3): 187-192.
(编辑 龙怀中)
基金项目:廊坊师范学院重点科研资助项目(LSZZ200902)
收稿日期:2009-08-25;修订日期:2010-01-15
通信作者:李爱昌,教授;电话:0316-2188370;E-mail:aichangli@hotmail.com


