Trans. Nonferrous Met. Soc. China 22(2012) 2898-2905
Oxidation behaviour of molten ZK60 and ME20 magnesium alloys with magnesium in 1,1,1,2-tetrafluoroethane/air atmospheres
CHEN Hu-kui, GONG Zan-fang
Department of Chemistry and Chemical Engineering, Baoji University of Arts and Science, Baoji 721013, China
Received 1 November 2011; accepted 10 September 2012
Abstract:
The oxidation behaviour of molten ZK60 and ME20 magnesium alloys in 1% 1,1,1,2-tetrafluoroethane/air atmospheres at 720 °C was compared with that of molten magnesium. The oxidation kinetics of these three melts was determined by thermograyimetric measuring instrument, and the surface films of the oxidized samples were examined by scanning electron microscope (SEM), X-ray diffractometry905hineseming; strain rates as: temperature (XRD) and X-ray photoelectron spectroscopy (XPS). The results show that the oxidation rate of molten ZK60 or ME20 alloys is much lower than that of molten magnesium in 1% 1,1,1,2-tetrafluoroethane/air atmospheres. The surface film formed on the molten magnesium is composed of MgF2, MgO and C, while the film formed on ZK60 melt mainly consists of MgF2, MgO, C and some ZrF4, and the film on ME20 mainly consists of MgF2, MgO, C and a small amount of CeF4. The good oxidation resistances of ZK60 and ME20 alloy melts may be caused by their major alloying elements Zr and Ce, respectively.
Key words:
ZK60 alloy; ME20 alloy; magnesium alloy melt; Mg melt; 1,1,1,2-tetrafluoroethane; alloying element; oxidation;
1 Introduction
Molten magnesium has an extremely high affinity for oxygen and high vapor pressure, which causes magnesium to oxidize rapidly and burn in air during the handling process. To address this issue, the melting and casting operation of magnesium and magnesium alloys is usually protected by the use of protective gases (sometimes referred to as “cover gases”) over the melts. Among the various cover gases, sulphur hexafluoride (SF6) is considered the optimal cover gas because of its non-toxic, non-corrosive and good protective effects, which has been widely used in the magnesium industry [1]. However, due to its extremely high greenhouse effect (the global warming potential is 23900) as well as a very long retention period in the atmosphere (3200 years), the use of SF6 is no longer acceptable environmentally, which has caused the magnesium industry to seek for alternatives to SF6 for magnesium melt protection [2-4]. Until now, it has been found that 1,1,1,2-tetrafluoroethane ( HFC-134a ) is a possible substitute for SF6 and HFC-134a can provide effective protection for magnesium and some magnesium alloy melts [5,6]. More recently, some efforts have been made to study the oxidation behaviour of molten magnesium and magnesium alloys in the atmospheres [7-10]. Moreover, some difference in oxidation behaviour between the melts has also been found. For example, LIU et al [8] compared the oxidation behaviour of molten AZ91D magnesium alloy with molten magnesium in HFC-134a/air atmospheres, and found that the oxidation rate of molten AZ91D magnesium alloy was lower than that of molten magnesium in the atmosphere of air containing more than 1% HFC-134a, which was considered to be related to the alloying element Al. Zirconium and cerium are also main alloying elements of magnesium alloys. Although it is reported that ZK60 magnesium alloy which contains alloying element Zr has good oxidation resistance in HFC-134a/air atmospheres [10]. However, the research on the comparison of the oxidation behaviours of molten magnesium alloys ZK60 and ME20 which contain alloying element Ce with molten magnesium in HFC-134a/air atmospheres has not been reported until now.
In this work, the oxidation resistances of molten magnesium alloys ZK60 and ME20 were compared with that of molten magnesium in 1% HFC-134a/air atmospheres at 720 °C. The objective was to understand the effect of alloying elements Zr and Ce on the oxidation behaviour of molten magnesium in HFC-134a/air atmospheres.
2 Experimental
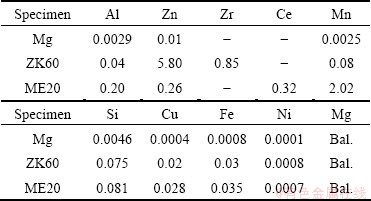
The main materials for oxidation tests were commercially pure magnesium, ZK60, ME20 magnesium alloys and HFC-134a gas. The chemical compositions of pure magnesium, ZK60 and ME20 magnesium alloys are shown in Table 1. The chemical composition of HFC-134a gas in mass fraction is HFC-134a≥99.8%, HCl≤0.0001%, H2O≤0.001%. The cylindrical samples with 50 mm in diameter and 3.0 mm in height for the study were cut from as-cast ingots. The samples were manually ground with 320 grit SiC paper and rinsed with acetone. In order to minimize the oxidation at room temperature exposure, the samples were immediately kept in a vacuum desiccator after rinsing.
Table 1 Chemical composition of magnesium and magnesium alloys (mass fraction, %)
Oxidation tests of molten magnesium and ZK60 and ME20 alloys in the atmosphere of HFC-134a/air were performed in a thermogravimetric measuring instrument. The instrument was composed of a recording electronic balance with an accuracy of 0.1 mg, a resistance furnace with sealing lid, a magnesia crucible and a gas supply system. Details about the experimental setup can be found in Ref. [7]. The magnesia crucible was heated to constant mass at 800 °C before oxidation test. The gas mixture of air and 1% HFC-134a, which were dried by passing them through the column of CaCl2 and silica-gel desiccant, was continuously introduced by the gas supply system into the hot chamber of the resistance furnace at 500 mL/min. After the gas mixture was passed into the chamber for 1 h, the samples of pure magnesium or ZK60 and ME20 magnesium alloys were placed in the crucible which had been suspended in the chamber and then heated to 720 °C at a rate of 50 K/min. The mass gains of the samples were continuously measured by the electronic balance when the samples were held in the atmosphere of air containing 1% HFC-134a at 720 °C for time intervals up to 150 min.
After oxidation treatment, the surface morphology of the samples was investigated using a scanning electron microscope (SEM). The phase composition of the surface film formed on the samples was identified by an X-ray diffractometer (XRD) with a Cu Kα source operated at 40 kV and 40 mA. The chemical composition and the chemical state of the surface film were determined by X-ray photoelectron spectroscopy (XPS) using Mg Kα radiation. The binding energies were calibrated by taking carbon C1s peak (285.0 eV) as reference. The measurement accuracy for the electron binding energy was about 0.2 eV.
3 Results
3.1 Oxidation kinetics
Figure 1 shows the mass gain versus time curves of molten magnesium, ZK60 and ME20 magnesium alloys in the atmospheres of air containing 1% HFC-134a at 720 °C. It can be seen that all the curves approximately follow a parabolic law, but the mass gains for different metals are much different. Molten magnesium has much greater mass gain than those of molten ZK60 and ME20 alloy. For the two magnesium alloy melts, the mass gain of ZK60 alloy is higher than that of ME20 alloy. This indicates that in the atmospheres of 1% HFC-134a/air, molten magnesium, ZK60 and ME20 alloys all have good oxidation resistances, but the oxidation resistances of the latter two alloy melts are higher than that of magnesium melt. The order of the oxidation resistances for the three materials is ME20> ZK60>Mg.
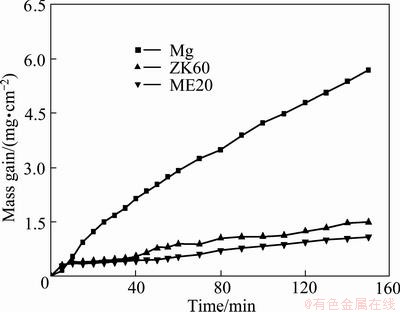
Fig. 1 Curves of mass gain versus time of molten magnesium and magnesium alloys oxidized in atmospheres of air containing 1% HFC-134a at 720 °C
3.2 Surface morphology
Figure 2 shows the SEM images of the surface of molten magnesium, ZK60 and ME20 magnesium alloys after exposure in air containing 1% HFC-134a at 720 °C for 2.5 h. The surface of molten magnesium, as observed in Fig. 2(a), is not very regular, and there are many irregularly distributed concavities and small white granules on the surface (the main composition of the white granules analyzed by EDS is O and Mg, which indicates that the white granules may be MgO), but it is still protective. The surface of ZK60 (Fig. 2(b)) becomes smoother and denser than magnesium and the white spots obviously reduce. For ME20 alloy, Fig. 2(c) shows that it has almost the same surface morphology as ZK60 alloy besides the less white spots. This indicates that the atmospheres of 1% HFC-134a in air can provide a satisfactory protection for magnesium, ZK60 and ME20 alloy melts, and the protection of the atmospheres for the latter two alloy melts is better than that for magnesium melt. This conclusion keeps highly consistent with the results of the oxidation kinetics above.
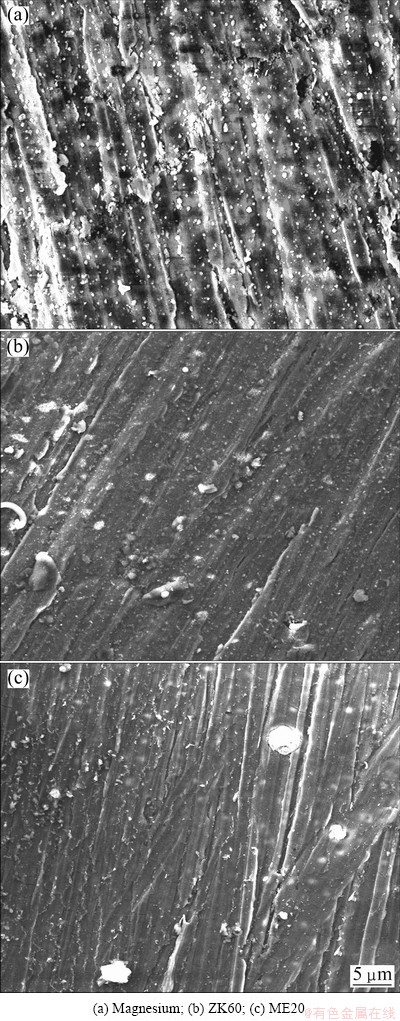
Fig. 2 Surface morphologies of magnesium and alloys after oxidation in air containing 1%HFC-134 at 720 °C
3.3 Surface phase composition
Figure 3 shows the XRD patterns of the surface films formed on molten magnesium, ZK60 and ME20 magnesium alloys in the atmosphere of air containing 1% HFC-134a. As can be seen, all the XRD patterns contain three groups of diffraction peaks corresponding to MgF2, Mg and graphite C, respectively, and MgF2 and Mg are the predominant crystalline phases. However, considering the very high reactivity of molten magnesium, Mg cannot exist in the surface films alone. So, the presence of Mg peaks in the all patterns may be caused by the X-ray radiation penetration of the surface films into the substrate metal. Therefore, the XRD analysis results suggest that the surface films formed on molten magnesium, ZK60 and ME20 magnesium alloys in 1% HFC-134a/air atmospheres are mainly composed of MgF2 with a small amount of C. However, surprisingly, we did not observe the peaks corresponding to MgO in our work although there was a large amount of oxygen present in the atmospheres.
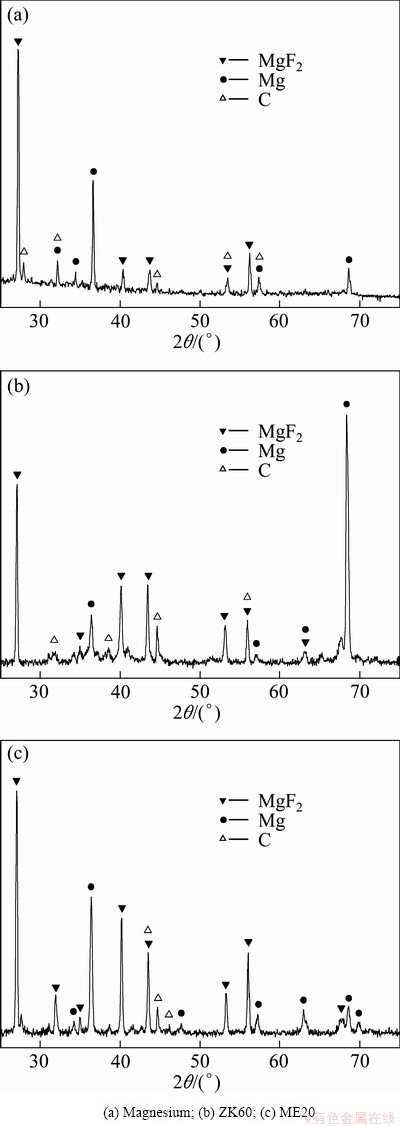
Fig. 3 XRD patterns of surface films formed on molten magnesium and alloys in air containing 1% HFC-134a

Fig. 4 XPS survey spectra of surface film formed on molten metals in air containing 1% HFC-134a at 720 °C
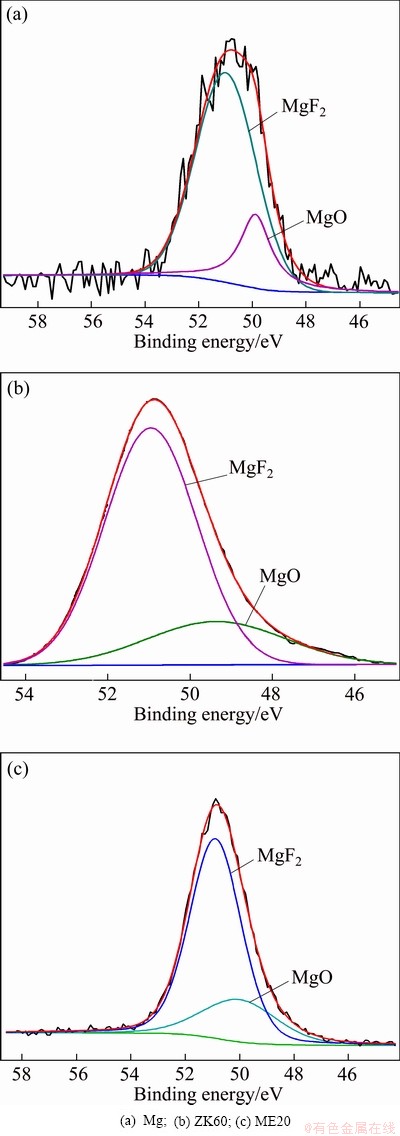
Fig. 5 XPS spectra of Mg 2p of surface film formed on molten metals in air containing 1% HFC-134a at 720 °C
Since magnesium has an extremely high affinity for oxygen, MgO should exist in the surface films formed on molten magnesium, ZK60 and ME20 magnesium alloys in the atmospheres of air containing 1% HFC-134a. In order to verify the existence of MgO and other minor phases in these surface films, the films were also examined by XPS. Figure 4 illustrates the XPS survey spectra of the films. The corresponding high-resolution Mg 2p, F 1s and C 1s XPS spectra of the films are given in Figs. 5-7, respectively, and the Zr 3d and Ce 3d XPS spectra are shown in Fig. 8. From Fig. 4, it can be seen that the surface film on molten magnesium contains Mg, F, O, and C elements, whereas the surface films on molten ZK60 and ME20 alloys, in addition to Mg, F, O, and C elements, also contains small amounts of Zr and Ce elements, respectively. The content of Mg and F elements in all the films is much higher than that of O and C elements and the level of O in the films of ZK60 and ME20 alloys is lower than that in the films of Mg. The Si, Ar and Na elements present in all the XPS survey spectra may be contaminants originating from the process of preparation and analysis of the samples. As shown in Fig. 5, the Mg 2p spectrum of the films on molten magnesium, ZK60 and ME20 alloys can be fitted to two components: the binding energy peak at (50.9± 0.2) eV is assigned to MgF2, the peak at (49.8±0.2) eV is attributed to MgO. The intensity of the peak at (50.9± 0.2) eV is greater than that of the peak at (49.8±0.2) eV, indicating that the content of MgF2 in the surface films is higher than that of MgO. In Fig. 6, the F 1s spectrum of the films on molten magnesium, ZK60 and ME20 alloys discloses a single peak at a binding energy of (685.5±0.2) eV, which is associated with MgF2 for molten Mg, or the co-existence of MgF2 and ZrF4 for molten ZK60 alloy, or the co-existence of MgF2 and CeF4 for molten ME20 alloy. Since the amount of Zr in ZK60 alloy and Ce in ME20 alloy is much lower than that of Mg, the ZrF4 content in the film formed on molten ZK60 alloy and the CeF4 content in the film formed on molten ME20 alloy are much lower than that of MgF2. Figure 7 shows that C1s spectrum of all the films is fitted to three different peaks. The peak at (284.9±0.2) eV is attributed to carbon in graphite and the peaks at (286.4±0.2) eV and (285.2±0.2) eV for ME20 alloy, and (287.3±0.2) eV and (288.5±0.2) eV for ME20 alloy are assigned to the chemisorbed CO and CO2, respectively, which result from the reaction of the C (a decomposition product of HFC-134a at high temperatures) with O2 in HFC-134a/air atmospheres. The graphite carbon content in the film is greater than that of CO and CO2. The Zr 3d spectrum (Fig. 8(b)) of the surface film formed on the molten ZK60 alloy and the Ce 3d spectrum (Fig. 8(c)) on the molten ME20 alloy are located at (185.5±0.2) eV and (880.0±0.2) eV, which corresponds to ZrF4 and CeF4, respectively.
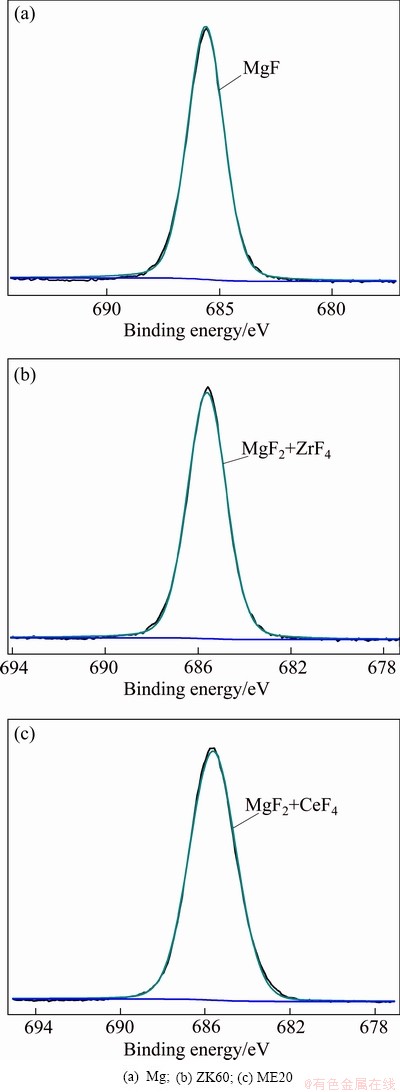
Fig. 6 XPS spectra of F 1s of surface film formed on molten metals in air containing 1% HFC-134a at 720 °C
The above XPS analysis results show that MgO exists in the surface films formed on molten magnesium and alloys in 1 % HFC-134a/air atmospheres, and other minor phases, such as ZrF4 and CeF4, are also present in the films.
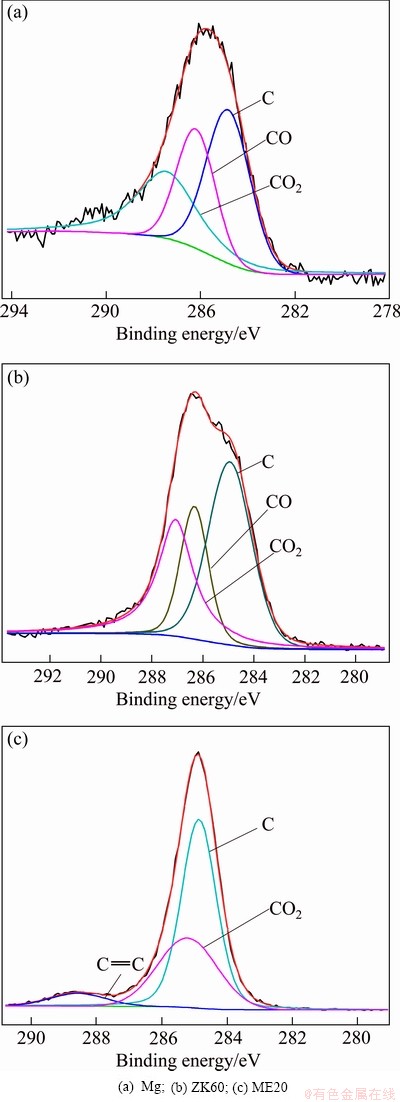
Fig. 7 XPS spectra of C 1s of surface film formed on molten metals in air containing 1% HFC-134a at 720 °C
The main reason why MgO and these minor phases were not detected by XRD in the surface films formed in air containing 1% HFC-134a may be that either these products are amorphous or their amounts are too little to produce a sufficient X-ray signal. Another reason may be that the high intensity signals from the bulk magnesium in all patterns make these minor phases in the surface film not be detected by XRD.

Fig. 8 XPS spectra of Zr 3d of surface film formed on molten ZK60 alloy (a) and XPS spectra of Ce 3d of surface film formed on molten ME20 alloy (b) in air containing 1% HFC-134a at 720 °C
Based on the results of XRD and XPS analyses above, it can be concluded that the surface film formed on molten magnesium in 1% HFC-134a/air atmospheres is composed of MgF2, MgO and C. The surface film formed on ZK60 melt in the atmospheres mainly consists of MgF2, MgO, C and some ZrF4, and the film on ME20 melt mainly consists of MgF2, MgO, C and small amounts of CeF4.
4 Discussion
It is known that when molten magnesium is exposed to fluorine-bearing atmospheres, its surface is covered with a protective magnesium fluoride/oxide film of few nanometers, and fluorine plays the key role in this film formation [5-9]. From the results obtained in the present study, it is found that after molten magnesium, ZK60 and ME20 magnesium alloys were exposed in the 1% HFC-134a/air atmospheres, the protective films composed of MgF2 and MgO as well as other minor phases were formed on their surface, which led to the oxidation of all the molten metals following the parabolic law. It is considered that the presence of large amounts of MgF2 in the surface films is the main reason that the films are protective, or 1% HFC-134a/air gas mixture can provide protection for the molten metals [7]. However, it is also found that there is a difference in oxidation rate between the molten metals. The difference in oxidation rate of the molten metals may be related to their alloying elements.
It has been found that the addition of small amounts of reactive element Zr, which has a very high oxygen affinity to magnesium alloys can improve their high temperature oxidation properties in air [11], while larger additions have the opposite effect [12]. From the results obtained in the present study, it can be seen that additions of small amounts of Zr to magnesium contribute to improve the oxidation resistance of molten magnesium in HFC-134a/air atmospheres. This is because Zr and Zn are the major alloying elements of ZK60 alloy, and the XPS analysis results above indicate that ZrF4 was present in the surface film formed on molten ZK60 alloy but the compound of Zn was not. Hence, it can be considered that the decrease of oxidation rate of molten ZK60 alloy in HFC-134a/air atmospheres compared with molten magnesium may be caused by its alloying element Zr. The presence of CeF4 and the absence of the Zn compounds can be explained as follows. In HFC-134a/air atmospheres, the following reactions between alloying elements Zr, Zn and F2 may take place:
Zr(l)+2F2(g)=ZrF4(s) (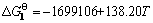 ) (1)
) (1)
Zn(l)+F2(g)=ZnF2(s) ( ) (2)
) (2)
Since the Gibbs free energy change of reaction (1) is far more negative than that of reaction (2) at 720 oC, according to the thermodynamic point of view, reaction (1) can occur and reaction (2) cannot take place actually, which means that ZrF4 can be detected by the XPS but ZnF2 cannot.
The role of Zr can be explained in terms of so called reactive element effect (REE) [11]. It is accepted [11] that the growth of a compact surface film is controlled by the solid-state diffusion of ions through the film. For the surface films on magnesium and its alloy, since the outward diffusion rate of Mg was much faster than that of the inward diffusion of oxygen [13,14], the growth of the film was controlled by the outward diffusion of Mg through the film [15]. According to the recent REE theory, the Zr in the film on molten ZK60 alloy diffuse to native magnesium oxide grain boundaries due to segregation and block the outward diffusion of Mg. Therefore, the growth of the film slowed down and a reduction in oxidation rate of molten ZK60 alloy was achieved.
The influence of Ce addition on the resistance to oxidation of magnesium alloy has been investigated. LIN et al [16] reported that the small addition of rare-earth element Ce into magnesium alloys AZ91D and AM50 could improve their oxidation resistance in air. However, a further research [17] found that the Ce addition to AM50 magnesium alloy has two opposite effects. To rapid solidification AM50 alloy, the resistance to oxidation of the alloy is reinforced. But when the alloy was prepared by slow solidification at the normal cooling rate, the detrimental effect was dominant. In this work, we found that the oxidation rate of molten ME20 magnesium alloy was much lower than that of molten magnesium. The surface film formed on molten ME20 alloy contained some CeF4, which was not present in the surface film formed on molten magnesium. The presence of CeF4 is due to the following reaction:
Ce(l)+2F2(g)=CeF4(s) (3)
These results imply that Ce additions to magnesium can also improve the oxidation resistance of molten magnesium in HFC-134a/air atmospheres. Because Ce is a kind of reactive element, it is highly probable that Ce in ME20 alloy has the same mechanism of action as Zr in ZK60 alloy.
5 Conclusions
1) The oxidation rates of molten ZK60 and ME20 alloys are much lower than that of molten magnesium in 1% HFC-134a/air atmospheres at 720 °C.
2) The surface film formed on molten magnesium in 1% HFC-134a/air atmospheres is composed of MgF2, MgO and C. However, the film formed on ZK60 melt in the atmospheres contains some ZrF4 besides MgF2, MgO and C, and the film on ME20 contains a small amount of CeF4 in addition to MgF2, MgO and C.
3) The significantly improved oxidation resistances of ZK60 and ME20 alloy melts are attributed to Zr and Ce in the surface films formed on the two melts, which diffuse to native magnesium oxide grain boundaries during the elevated temperature oxidation and block Mg diffusion through the films.
References
[1] CASHION S P, RICKETTS N J, HAYES P C. Characterisation of protective surface films formed on molten magnesium protected by air/SF6 atmospheres [J]. Journal of Light Metals, 2002, 2: 37-42.
[2] BARTOS S, KANTAMANENI R. Global magnesium industry charts its own course for climate protection [C]// Proceedings of the 60th Annual International Magnesium Association Conference. Stuttgart, GER: International Magnesium Association, 2003: 23-25.
[3] ERICKSON S C, KING J F, MELLERUD T. Conserving SF6 in Mg melting operation [J]. Foundry Management and Technology, 1998, 126(6): 38-45.
[4] GJESTLAND H, WESTENGEN H, PLAHTE S. Use of SF6 in the magnesium industry: An environmental challenge [C]// Proceedings of the Third International Magnesium Conference. Manchester, UK: The Institute of Materials, 1996: 33-41.
[5] RICKETTS N J, CASHION S P. Hydrofluorocarbons as cover gases for magnesium melt protection [C]// Magnesium Technology 2001. Warrendale, USA: The Minerals Metals & Materials Society, 2001: 31-36.
[6] CASHION S P, RICKETTS N J, FROST M T, KORN C J. The protection of molten magnesium and its alloys during die-casting [C]// The 8th Annual IMA Magnesium in Automotive Seminar. Aalen, GER: International Magnesium Association, 2000: 12-14.
[7] CHEN H K, LIU J R, HUANG W D. Oxidation behavior of molten magnesium in air/HFC-134a atmospheres [J]. Journal of Materials Science, 2006, 41: 8017-8024.
[8] LIU J R, CHEN H K, ZHAO L, HUANG W D. Oxidation behaviour of molten magnesium and AZ91D magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres [J]. Corrosion Science, 2009, 51: 129-134.
[9] WON H, KIM Y J. Effects of cover gases on melt protection of Mg alloys [J]. Journal of Alloys and Compounds, 2006, 422: 208-213.
[10] CHEN H K, LIU J R, HUANG W D. The protective surface film formed on molten ZK60 magnesium alloy in 1,1,1,2-tetrafluoroethane/air atmospheres [J]. Corrosion Science, 2010, 52: 3639-3645.
[11] CZERWINSKI F. The early stage oxidation and evaporation of Mg-9%Al-1%Zn alloy [J]. Corrosion Science, 2004, 46: 377-386.
[12] PEREZ P, GARCES G, ADEVA P. Oxidation behavior of a PVD-processed Mg-10.6Zr alloy [J]. Oxidation of Metals, 2002, 58(5-6): 607-621.
[13] SALAS O, NI H, JAYARAM V, VLACH K C, LEVI C G, MEHRABIAN R. Nucleation and growth of Al2O3/Metal composites by oxidation of aluminum alloys [J]. Journal of Materials Research, 1991, 6: 1964-1981.
[14] ZAYAN M H. Model for non-protective oxidation of Al-Mg alloys [J]. Oxidation of Metals, 1990, 34: 465-472.
[15] ZENG X Q, WANG Q D, LU Y Z, DING W J, ZHU Y P, ZHAI C Q, LU C, XU X P. Behavior of surface oxidation on molten Mg–9Al–0.5Zn–0.3Be alloy [J]. Materials Science and Engineering A, 2001, 301: 154-161.
[16] LIN P Y, ZHOU H, LI W, LI W P, SUN N, YANG R. Interactive effect of cerium and aluminum on the ignition point and the oxidation resistance of magnesium alloy [J]. Corrosion Science, 2008, 50: 2669-2675.
[17] LIN P Y, ZHOU H, SUN N, LI W P, WANG C T, WANG M X, GUO Q C, LI W. Influence of cerium addition on the resistance to oxidation of AM50 alloy prepared by rapid solidification [J]. Corrosion Science, 2010, 52: 416-421.
ZK60和ME20镁合金熔体与镁熔体在HFC-134a/空气气氛中的氧化行为
陈虎魁,弓赞芳
宝鸡文理学院 化学化工系,宝鸡 721013
摘 要:对镁合金ZK60和ME20熔体与镁熔体在1%HFC-134a/空气气氛中的高温氧化行为进行比较。使用热重仪测定这3种熔体的氧化动力学曲线,使用扫描电镜观察氧化后样品表面的形貌,并用X射线衍射仪和X射线光电子能谱仪分析样品表面的相成分。结果表明:ZK60和ME20镁合金熔体在1% HFC-134a/空气气氛中的氧化速度远远低于镁熔体的氧化速度;镁熔体表面所形成的氧化膜主要由MgF2、MgO和C组成,而ZK60和ME20镁合金熔体表面所形成的氧化膜除了含有MgF2、MgO和C以外,还分别含有少量的ZrF4和CeF4。ZK60和ME20镁合金熔体在该气氛中氧化阻力的提高分别与它们的合金元素Zr和Ce有关。
关键词:ZK镁合金;ME20镁合金;镁合金熔体;镁熔体;HFC-134a;合金元素;氧化
(Edited by LI Xiang-qun)
Foundation item: Project (SJ08-ZT05) supported by the Natural Science Basic Research Plan in Shaanxi Province of China; Project (ZK1050) supported by the Key Scientific Research Plan of Baoji University of Arts and Science, China
Corresponding author: CHEN Hu-kui; Tel/Fax: +86-917-3536630; E-mail: hkchen7115@yahoo.com.cn
DOI: 10.1016/S1003-6326(11)61548-3
Abstract: The oxidation behaviour of molten ZK60 and ME20 magnesium alloys in 1% 1,1,1,2-tetrafluoroethane/air atmospheres at 720 °C was compared with that of molten magnesium. The oxidation kinetics of these three melts was determined by thermograyimetric measuring instrument, and the surface films of the oxidized samples were examined by scanning electron microscope (SEM), X-ray diffractometry905hineseming; strain rates as: temperature (XRD) and X-ray photoelectron spectroscopy (XPS). The results show that the oxidation rate of molten ZK60 or ME20 alloys is much lower than that of molten magnesium in 1% 1,1,1,2-tetrafluoroethane/air atmospheres. The surface film formed on the molten magnesium is composed of MgF2, MgO and C, while the film formed on ZK60 melt mainly consists of MgF2, MgO, C and some ZrF4, and the film on ME20 mainly consists of MgF2, MgO, C and a small amount of CeF4. The good oxidation resistances of ZK60 and ME20 alloy melts may be caused by their major alloying elements Zr and Ce, respectively.


