Thermodynamic modeling of lead blast furnace
TAN Peng-fu(谭鹏夫)
(Mount Isa Mines Limited, Queesland 4825, Australia)
Abstract:
A thermodynamic model was developed to predict the distribution behavior of Cu, Fe, S, O, Pb, Zn, As, and the heat balance in a lead blast furnace. The modeling results are validated by the plant data of a lead smelter in Kazakhstan. The model can be used to predict any set of controllable process parameters such as feed composition, smelting temperature, degree of oxygen enrichment and volume of oxygen-enriched air. The effects of the blast air, industrial oxygen, and coke charge on the distribution of Cu, Fe, S, O, Pb, Zn, As, the heat balance, and the lead loss in slag, were presented and discussed.
Key words:
thermodynamic model; lead blast furnace; lead smelting CLC number: TF812;
Document code: A
1 INTRODUCTION
Thermodynamic models can help engineers to better understand the relationship between the operating parameters and products, and to optimize the operating conditions of the furnaces. The author and his co-worker have developed thermodynamic models of nickel process[1, 2], copper process[3], direct lead process[4], and dioxin formation in thermal processes[5, 6]. Many metallurgical processes, such as nickel flash smelting, nickel direct high-grade matte smelting, copper Isasmelt, copper flash smelting, Mitsubishi copper smelting, Noranda copper smelting, QSL lead smelting, KIVCET lead smelting, and dioxin formation in iron ore sintering have been simulated.
Several different approaches to lead blast furnace have been reported[7-10]. But the distributions of main elements in lead blast furnace have not been understood well. In this work, a thermodynamic model of a lead blast furnace is developed to assist in understanding of the process chemistry and optimizing the performance of blast furnace. FactSage software[11] has been used in the work.
2 PROCESS PARAMETERS
The compositions of the sinter and coke, which were used in the blast furnace at this lead smelter in 2003, are listed in Tables 1 and 2, respectively. The volume of blast air and the amount of coke charge, and heat loss are shown in Table 3.
3 VALIDATION OF THERMODYNAMIC MODEL
These parameters are input into the thermo-dynamic model. The modeling results are compared with the plant data of 2003, and the comparison is shown in Table 4. It shows that the modeling results for the slag and lead bullion are in good agreement with the plant data.
Table 1 Mineralogical compositions of sinter
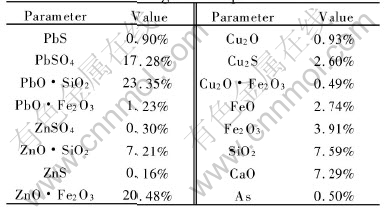
Table 2 Compositions of coke
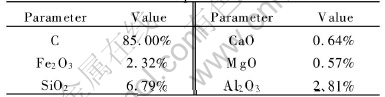
Table 3 Operating parameters of lead blast furnace

According to Table 4, the prediction of matte phase is not very good, but it has only little effect
Table 4 Comparison between modeling results and plant data of 2003 (mass fraction, %)
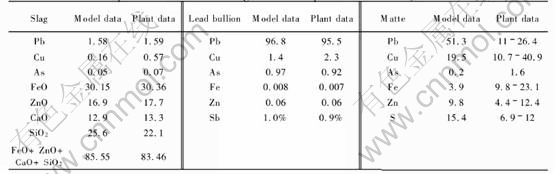
on the precision of the whole modeling results.
4 MODELING RESULTS AND DISCUSSION
4.1 Effect of coke on charge
The modeling results for the different coke on charges at constant blast air (Flow rate of blast air is 30000m3/h, flow rate of industrial oxygen is 500m3/h) are shown in Figs.1-3.
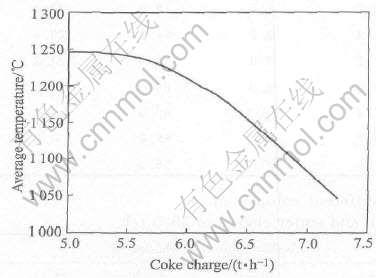
Fig.1 Effect of coke charge on average temperature of main reaction area blast air and industrial oxygen
A decrease of coke on charge will lead to an increase of the smelting temperature, as shown in Fig.1, as the reactions 2PbO(s)+C=2Pb(l)+CO2 and 2Fe2O3(s)+C=4FeO(l)+CO2 are endothermic. A decrease of the coke on charge also results in an increase in lead content of slag and the distribution of lead to slag (or the lead loss in the slag), and a decrease of the lead distribution to lead bullion (or the recovery rate of lead), as shown in Figs.2 and 3.
4.2 Effect of blast air
The modeling results for different blast air rates and four levels of coke on charge (6.3, 6.0, 5.7 and 5.4t/h) are shown in Tables 5-8, respectively.
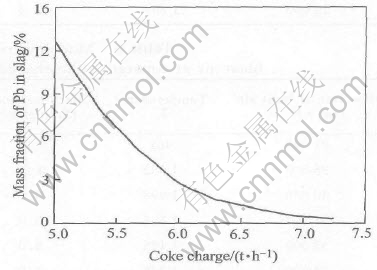
Fig.2 Effect of coke charge on content of lead in slag at constant flow rae of when blast air and industrial oxygen
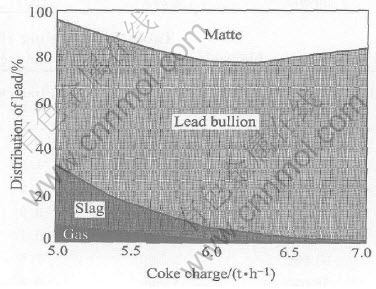
Fig.3 Effect of coke charge on distribution of lead in matte, lead bullion, slag and off-gas at constant flow rate blast air and industrial oxygen
When coke on charge is constant, an increase in blast air rate will lead to the results as follows. 1) An increase in the smelting temperature, as shown in Fig.4. 2) An increase of the content of lead in the slag, as shown in Fig.5. 3) An increase of the distribution of lead in the slag (or the lead loss in the slag), and a decrease of the lead distri-
Table 5 Modeling results for different volumes of blast air at constant coke charge of 6.3t/h and sinter charge of 38.5t/h
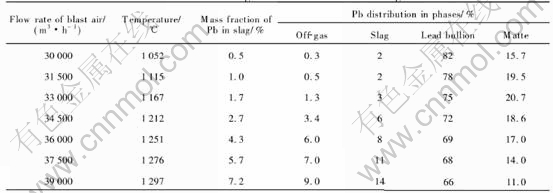
Table 6 Modeling results for different volumes of blast air at constant coke charge of 6.0t/h and sinter charge of 38.5t/h
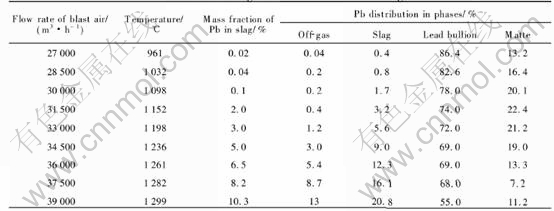
Table 7 Modeling results for different volumes of blast air at constant coke charge of 5.7t/h and sinter charge of 38.5t/h
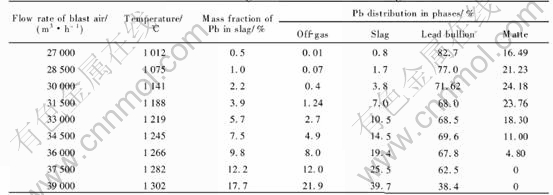
bution in the lead bullion (or the recovery rate of lead), as shown in Tables 5-8.
4.3 Optimization of coke charge
Three parameters will be considered for the optimizations of coke charge as follows. 1) The average smelting temperature should not be below 1100℃. Otherwise some solid solutions will precipitate from the liquid slag, will increase the slag viscosity, and could result in the difficulty of the slag tapping. 2) The lead content in slag and the distribution of lead in slag should be low. 3) The distribution of lead in lead bullion should be high.
The optimizing results are summarized in Tables 5-8, and are shown in Table 9. The pre-sent coke charge is 6.3t/h, and it could be decreas-
Table 8 Modeling results for different volumes of blast air at constant coke charge of 5.4t/h and sinter charge of 38.5t/h
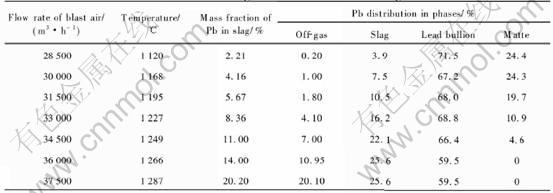
Table 9 Optimizing results for lead blast furnace(sinter charge: 38.5t/h)
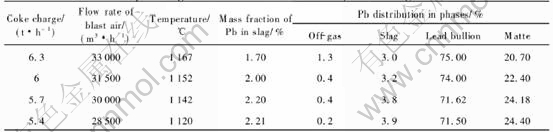
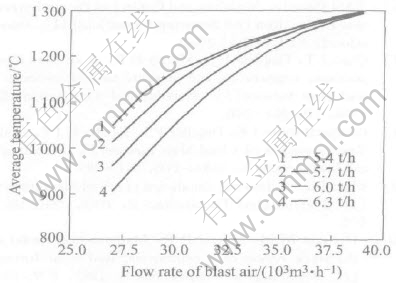
Fig.4 Effect of flow rate of blast air on average temperature of main reaction area
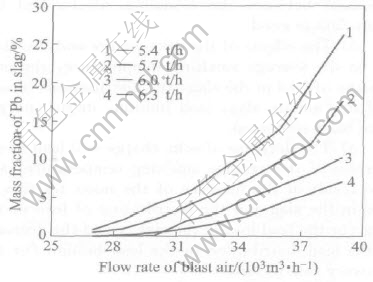
Fig.5 Effect of flow rate of blast air on lead in slag
ed to 5.7-6t/h. At the same time, the blast air should be decreased accordingly, as shown in Table 9. If the coke charge is decreased to 5.4t/h, the smelting temperature could be too low, and some tapping problem of slag could occur.
4.4 Effect of amount and quality of coke
When the coke on charge decreases and the lead content in slag is held constant at 2%, the relation between coke on charge and average smelting temperature in the reaction zone is shown in Fig.6.
When the percentage of lead in slag is held constant at 2%, a decrease of coke on charge will lead to a decrease of the smelting temperature, as shown in Fig.6. If the smelting temperature is too low, the slag could be difficult to be tapped because of the high viscosity.
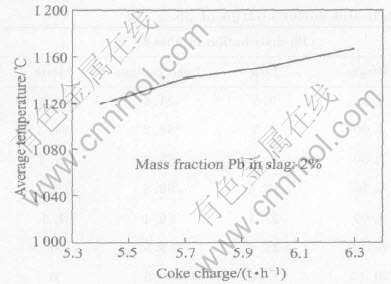
Fig.6 Effect of coke charge on average temperature of main reaction area
There exists difference between Fig.1 and Fig.6 should be noted. In Fig.1, a decrease in coke on charge will lead to an increase in the smelting temperature when the blast air is constant. In Fig.6, a decrease of coke on charge will lead to a decrease in the smelting temperature when the lead content in slag is held constant at 2%.
The quality of coke is also an important parameter for the operations. Poor coke quality will result in reduced smelting temperature, and slag tapping difficulties.
5 CONCLUSIONS
1) The thermodynamic model of lead blast furnace is developed, and the model is validated against the plant data from the lead smelter. The agreement between the modeling results and the plant data is good.
2) The effects of the coke charge and the blast air on the average smelting temperature, the percentage of lead in the slag, the distributions of lead and zinc among slag, lead bullion, matte and gas have been simulated.
3) The decrease of coke charge will lead to the increase of the average smelting temperature, and also result in the increase of the mass fraction of lead in the slag and the distribution of lead in the slag (or the lead loss in the slag), and the decrease of the lead distribution in the lead bullion (or the recovery rate of lead).
4) The increase of flow rate of the blast air will lead to the increase of the smelting temperature, and result in the increase of the content of[CM(22]lead in the slag. The increase of volume of the[CM)]blast air will also lead to the increase of the distribution of lead in the slag (or the lead loss in the slag), and the decrease of the lead distribution in the lead bullion (or the recovery rate of lead).
REFERENCES
[1]TAN Peng-fu, Neuschuetz D. A thermodynamic model of nickel smelting and direct high-grade nickel matte smelting processes—Model development and validation[J]. Metallurgical and Materials Transactions B, 2001, 32B: 341-352.
[2]TAN Peng-fu, Neuschuetz D. A thermodynamic model of nickel smelting and direct high-grade nickel matte smelting processes—Distribution behaviors of Ni, Cu, Co, Fe, As, Sb and Bi[J]. Metallurgical and Materials Transactions B, 2001, 32B: 353-362.
[3]TAN Peng-fu, ZHANG Chuan-fu. Modeling of accessory element distribution in copper smelting process[J]. Scandinavian Journal of Metallurgy, 1997, 26(3): 115-122.
[4]TAN Peng-fu, ZHANG Chuan-fu. Thermodynamic modeling for direct lead processes[A]. Mishra B. EPD Congress 1998[C]. San Antonio, Texas: The Minerals, Metals and Materials Society (TMS). 1998. 815-820.
[5]TAN Peng-fu, Hurtado I, Neuschuetz D, et al. Thermodynamic modeling of PCDD/Fs formation in thermal processes[J]. Environmental Science and Technology, 2001, 35(9): 1867-1874.
[6]TAN Peng-fu. Modeling and Control of Dioxin Formation During Iron Ore Sintering Operations[M]. Duesseldorf: VDI Verlag, 2002.
[7]Chao J T, Dugdale P J, Morris D R, et al. Gas composition, temperature and pressure measurements in a lead blast furnace[J]. Metallurgical Transactions B, 1978, 9B: 293-300.
[8]Cowperthwaite J E, Dugdale P J, Landry C J F, et al. Energy aspects of a lead blast furnace[J]. Metallurgical Transactions B, 1980, 11B: 291-299.
[9]Chao J T. A dynamic simulation of a lead blast furnace[J]. Metallurgical Transactions B, 1981, 12B: 385-402.
[10]Hussain M M, Morris D R. Mathematical model of the stack region of a commercial lead blast furnace[J]. Metallurgical Transactions B, 1989, 20B: 97-106.
[11]Bale C W, Chartrand P. FactSage thermochemical software and databases[J]. CALPHAD, 2002, 26: 189-228.
Received date: 2004-09-29; Accepted date: 2004-11-15
Correspondence: TAN Peng-fu, Principal Scientist, PhD; E-mail: tanpengfu@yahoo.com


