
Pressure acid leaching of zinc sulfide concentrate
GU Yan(古 岩), ZHANG Ting-an(张廷安), LIU Yan(刘 燕), MU Wang-zhong(牟望重),
ZHANG Wei-guang(张伟光),DOU Zhi-he(豆志河), JIANG Xiao-li(蒋孝丽)
Key Laboratory of Ecological Utilization of Multi-metal Intergrown Ores of Ministry of Education,School of Materials and Metallurgy, Northeastern University, Shenyang 110004, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract:
Effects of particle size of the zinc sulfide concentrate, leaching temperature, solid-to-liquid ratio and additive amount on pressure acid leaching process of the zinc sulfide concentrate were studied. The results indicate that the additive can improve the reaction kinetics and the conversion rate. And sulfur can be successfully separated from the zinc sulfide concentrate as elemental sulfur. The reasonable experiment parameters are obtained as follows: the leaching temperature 150 ℃, oxygen partial pressure 1 MPa, additive amount 1%, solid-to-liquid ratio 1:4, leaching time 2 h, initial sulfuric acid concentration 15%, and particle size less than 44 μm. Under the optimum conditions, the leaching rate of the zinc can reach 95% and the reduction rate of the sulfur can reach 90%.
Key words:
zinc sulfide concentrate; pressure acid leaching; zinc; sulfur;
____________________________________________________________________________________________
1 Introduction
Zinc exists in the earth crust predominantly as sulfides, and sphalerite is its most important ore. There are 89 million tons recoverable deposits and 33 million tons industrial reserves in China[1-4]. China owns the most zinc ore in the world[2, 5-8]. Zinc sulfide concentrate is the main raw material for extracting zinc. Many investigations have been reported for the beneficiation of zinc ore to prepare concentrate from which zinc metal is produced by hydrometallurgical process. These processes involve a roasting step, which evolves toxic SO2 gas and requires a sulfuric acid plant to be set up in the smelter. Direct pressure leaching has several problems associated with maintenance of autoclave[9-13]. Among the alternative processes to treat the sphalerite, the hydrometallurgical route without pretreatment, such as direct oxidative leaching, is considered to be quite attractive[6, 14-15]. The pressure leaching has been commercially used in the metal ores and concentrates. It offers the advantages of more precise control, higher mineral utilization, and increased flexibility[16].
The present work reports such a study carried out on zinc sulfide concentrate, with high metal impurities. The process is based on a direct leaching with oxygen of bulk flotation concentrate.
2 Experimental
2.1 Materials
The feed for this study was zinc sulfide concentrate. The bulk concentrate from this ore was produced at Huludao Zinc Plant, Liaoning Province, China.
Tables 1 and 2 show the chemical compositions of the feed and the mineralogical analysis results of bulk concentrate, respectively. The major minerals present in the concentrate were sphalerite (ZnS), marmatite (ZnFeS), quartz (SiO2), and other minerals including Pb and Ti. Fig.1 shows the XRD pattern of the zinc sulfide concentrate.
Table 1 Chemical composition of ZnS concentrate (mass fraction, %)
![]()
Table 2 Mineralogical composition of head sample (mass fraction, %)
![]()
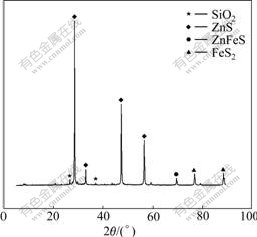
Fig.1 XRD pattern of zinc sulfide concentrate
Representative samples were used in all experiments. All chemicals used were of analytical grade and all solutions were made with distilled water. Metal content was analyzed by atomic absorption spectrometry (Perkin-Elmer).
2.2 Equipment
The leaching was carried out on laboratory scale.
Leaching was done in a pressure reactor called autoclave. Table 3 shows the leaching experiment equipments, and Table 4 shows the major technical parameters of the autoclave.
Table 3 Experimental equipments
Table 4 Related technical parameters of WHFS-2T high pressure reactor

3 Results and discussion
3.1 Leaching mechanism
In this leaching system, ZnS and sulfate inter-reacted and generated S, ZnSO4, H2O, etc.
In the absence of oxygen, the partial pressure of H2S formed slows down the kinetics of the leaching reaction. The addition of oxygen in the system reduces this effect:
ZnS+H2SO4+1/2O2=ZnSO4+S0+H2O↑ (low-iron A/C leaching) (1)
ZnS+H2SO4=ZnSO4+H2S (2)
H2S+Fe2(SO4)3=2FeSO4+H2SO4+S0 (3)
ZnS+Fe2(SO4)3=2FeSO4+ZnSO4+S0 (4)
2FeSO4+H2SO4+1/2O2=Fe2(SO4)3+H2O (5)
3.2 Effect of leaching temperature
The leaching temperature is an important thermodynamic parameter. The effect of leaching temperature on the leaching of zinc concentrate was studied at constant initial sulfuric acid concentration (15%), leaching temperature (150 ℃), solid-to-liquid ratio (1:4), particle size (<53 ?m), leaching time (1.5 h), oxygen partial pressure (0.8 MPa), stirring speed (480 r/min) and additive amount (1%).
Fig.2 shows increase in zinc and sulfur recovery with the increase in leaching temperature. When the leaching temperature was above 120 ℃, the slope of the curve was decreased. It could be conducted that 150 ℃ was the suitable leaching temperature.
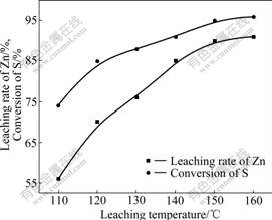
Fig.2 Effect of leaching temperature
3.3 Effect of oxygen partial pressure
In the process of pressure leaching, oxygen was an important reactive substance introduced into reacting system. The effect of oxygen partial pressure on the leaching of zinc concentrate was studied at constant initial sulfuric acid concentration (15%), leaching temperature (150 ℃), solid-to-liquid ratio (1:4), particle size (<53 ?m), leaching time (1.5 h), stirring speed (480 r/min) and additive amount (1%).
Fig.3 shows that the higher pressure made the higher leaching rate of zinc and conversion of sulfur, but in the range of high oxygen partial pressure, the increasing trend was slow. So, it could be constructed that 1 MPa was the best pressure for leaching of zinc sulfide concentrate.
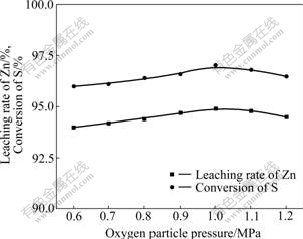
Fig.3 Effect of oxygen partial pressure on leaching
3.4 Effect of additive amount
The defreezed sulfur had strong wetting function, and it could be encapsulated over the surface of the concentrate, so as to hinder the contact between the concentrate and reaction medium. To prevent the concentrate particles to be encapsulated, the lignosulfonate as active agent was added into the leaching system.
The effect of additive amount on the leaching of zinc concentrate was studied at constant initial sulfuric acid concentration (15%), leaching temperature (150 ℃), solid-to-liquid ratio (1:4), particle size (<53 ?m), leaching time (1.5 h), stirring speed (480 r/min) and oxygen particle pressure (1 MPa).
Fig.4 shows that at 150 ℃, the leaching rate of zinc was 74.5%, while the conversion of sulfur was 29.8%, when there was no additive. For the surface hydrophobicity of the sulfide concentrate, it was unease to be wetted by water but by defreezed sulfur, and the non-oxidative sulfides were wetted and encapsulated in priority by sulfur in the process of pressure leaching. This increased the diffusion resistance and hindered the leaching of zinc sulfide concentrate seriously. With increasing the amount of additive, the surface tension of fluid sulfur was decreased, and the sulfur dropped from the surface of sulfide while stirring. However, when the amount of additive was over 0.8%, the leaching rate changed slowly. So, it could be concluded that 1% was the suitable additive amount.
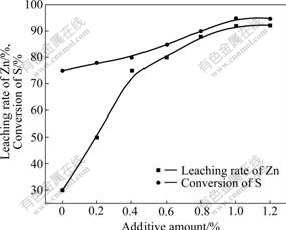
Fig.4 Effect of additive amount on leaching
3.5 Effect of solid-to-liquid ratio
The effect of solid-to-liquid ratio on the leaching of zinc concentrate was studied at constant initial sulfuric acid concentration (15%), leaching temperature (150 ℃), additive amount (1%), particle size (<53 ?m), leaching time (1.5 h), stirring speed (480 r/min) and oxygen particle pressure (1 MPa).
Fig.5 shows that, with increasing the amount of sulfate, the leaching rate of zinc and the conversion of sulfur were increased. When the solid-to-liquid ratio was over 1:4, the curve changed to be steady. However, solid-to-liquid ratio was small, the amount of acid was too small, and the leaching rate was low. There was no significance for leaching when the amount of sulfate increased. So, the suitable condition of solid-to-liquid ratio was 1:4.
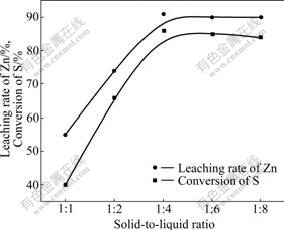
Fig.5 Effect of solid-to-liquid ratio on leaching
3.6 Effect of leaching time
The effect of leaching time on the leaching of zinc concentrate was studied at constant initial sulfuric acid concentration (15%), additive amount (1%), particle size (<53 ?m), solid-to-liquid ratio (1:4), leaching temperate (150 ℃), stirring speed (480 r/min) and oxygen particle pressure (1 MPa).
Fig.6 shows that with increasing the leaching time, leaching rate increased; but when the leaching time was over 1.5 h, the curve of leaching rate was changed to be steady. So, the suitable leaching time is 2 h.
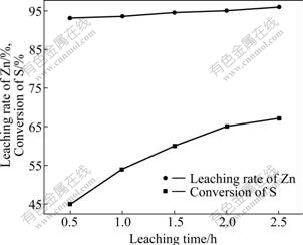
Fig.6 Effect of leaching time on leaching
3.7 Effect of particle size
The effect of leaching time on the leaching of zinc concentrate was studied at constant initial sulfuric acid concentration (15%), additive amount (1%), leaching time (2 h), solid-to-liquid ratio (1:4), leaching temperature (150 ℃), stirring speed (480 r/min) and oxygen particle pressure (1 MPa).
Fig.7 shows that the effect of the particle size on leaching process was serious. With decreasing the particle size, the leaching rate of zinc and conversion of sulfur were increased. The curve changed to be steady when the particle size was smaller than 44 ?m.
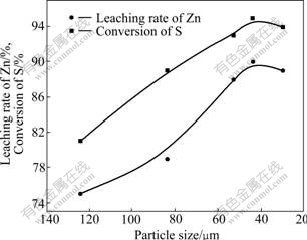
Fig.7 Effect of concentrate particle size on leaching
The definition formula of specific surface area is
 (6)
(6)
where S is the total surface area of the particles; m is the total mass of the particles; r is the radius of the particles; and ρ is the density of the particles.
The specific surface area and single particle size are in inverse relation. The pressure leaching of zinc sulfide concentrate is a liquid-solid reaction in autoclave. With decreasing the particle radius in unit volume, the probability of contract between the particle of concentrate and reaction medium is increased, which can improve the kinetic condition and increase the leaching rate of the reaction correspondingly.
4 Conclusions
1) This laboratory-scale investigation demonstrated the feasibility of using pressure leaching process to extract zinc and elemental sulfur from zinc sulfide concentrate. However, there are some areas that need further exploration to determine the relative merits of this process as compared with those methods already in place or put forward.
2) The reasonable experiment parameters are: leaching temperature 150 ℃, oxygen partial pressure 1 MPa, additive amount 1%, solid-to-liquid ratio 1:4, leaching time 2 h, initial sulfuric acid concentration 15%, and particle size smaller than 44 μm. The leaching rate of the zinc can reach 95% and the reduction rate of the sulfur can reach 90% at this condition.
3) Adding the additive can further enhance the leaching kinetics. Autoclave leaching of the concentrate allows for high zinc extraction with greatly decreased reaction time. The laboratory process shows sufficient promise for a detailed process development.
References
[1] WANG Hai-bei, JIANG Kai-xi. Study on the new process of pressure leaching of zinc confide concentrate [J]. Non-ferrous Metallurgical (Smelting Part), 2004(5): 2-4. (in Chinese)
[2] ?OPER M, ?ZMETIN C, ELIF ?, KOCAKERIM M M. Optimization study of the leaching of roasted zinc sulphide concentrate with sulphuric acid solutions [J]. Chemical Engineering and Process, 2004, 43: 1007-1014.
[3] SAHU S K, SAHU K K, PANDEY B D. Leaching of zinc sulfide concentrate from the Ganesh-Himal deposit of Nepal [J]. Metallurgical and Materials Transactions, 2006, 37B: 541-549.
[4] FRANAY J. Leaching of oxidized zinc ores in various media [J]. Hydrometallurgy, 1985, 15(2): 243-248.
[5] DONG Qiao-long. Comparison between zinc concentrate normal pressure leaching and high-pressure leaching [J]. China Non-ferrous Metallurgical, 2007, 8(4): 24-26. (in Chinese)
[6] HU Jian-dong, XU Ming, JIANG Jun-yang. Study on the leaching of zinc sulfide concentrate by catalyst and oxidation [J]. Non-ferrous Metal (Smelting Part), 2003(5): 21-23. (in Chinese)
[7] AKCIL A, CIFTCL H. Metals recovery from multi metal sulphide concentrates (CuFeS2-PbS-ZnS): Combination of thermal process and pressure leaching [J]. Int J Miner Process, 2003, 71: 233-246.
[8] AKCIL A. A preliminary research of acid pressure leaching of pyretic copper ore in Kure Copper Mine, Turkey [J]. Miner Eng, 2002, 15(12): 1193-1197.
[9] AKCIL A, CILTCL H. A study of the selective leaching of complex sulfides from the Eastern Black Sea region, Turkey [J]. Miner Eng, 2002, 15(6): 457-459.
[10] WANG Ji-kun, LI Cun-xiong, LI Yong, ZHANG Hong-yao, HUANG Pen, YAN Jiang-feng, LIU Lu, WEI Chang. The ε-pH figure of ZnS-FeS-H2O system during acid leaching under pressure of high-iron sphalerite [J]. Non-ferrous Metallurgy (Smelting Part), 2006(2): 2-5. (in Chinese)
[11] WANG Ji-kun, ZHOU Yan-xi, WU Ji-mei. Study on high iron containing sphslerite concentrate by acid leaching under pressure [J]. Non-ferrous Metallurgy (Smelting Part), 2004(1): 5-8. (in Chinese)
[12] GAO Liang-bin, HE Ji-cheng, HONG Hong-jiang. The technology of zinc sulphide concentrate in high-temperature and high-pressure leaching [J]. Non-ferrous Mining and Metallurgy, 2007, 23(4): 33-36. (in Chinese)
[13] WEERT G V, BOERING M. Selective pressure leaching of zinc and manganese from natural and man-made spinels using nitric acid [J]. Hydrometallurgy, 1995, 39: 201-213.
[14] PARKER E G. Oxidative pressure leaching of zinc concentrates [J]. CIM Bull, 1987, 74(829): 145-150.
[15] XU Zhi-feng, QIU Ding-fan, LU Hui-min, WANG Hai-bei. Review on research of oxidic-acidic pressure leaching of zinc concentrates [J]. Nonferrous Metals, 2005, 57(2): 101-105. (in Chinese)
[16] YANG Zhong-ping, JIN Xiao-zhu, HUANG Jian-hui. Extraction of zinc concentrates under oxygen stressing and acid leaching [J]. Mineral Resources and Geology, 2005, 8: 449-451. (in Chinese)
_____________________
Foundation item: Project(20050145029) supported by the Research Fund for the Doctoral Program of Higher Education of China; Project(2005221012) supported by Science and Technology Talents Fund for Excellent Youth of Liaoning Province, China
Corresponding authors: ZHANG Ting-an; Tel: +86-24-83687732; E-mail: zta2000@163.net; GU Yan; Tel: +86-13604054825; E-mail: 18512019@ qq.com
(Edited by YANG Bing)


