J. Cent. South Univ. (2012) 19: 9-16
DOI: 10.1007/s11771-012-0965-x![]()
Influence of zinc on electrochemical discharge activity of Mg-6%Al-5%Pb anode
WANG Nai-guang(王乃光), WANG Ri-chu(王日初), PENG Chao-qun(彭超群), FENG Yan(冯艳)
School of Materials Science and Engineering, Central South University, Changsha 410083, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2012
Abstract:
Mg-6%Al-5%Pb (mass fraction) anodes with different contents of zinc were prepared by melting and casting. The electrochemical discharge behavior of these anodes in 3.5% NaCl solutions was investigated by galvanostatic test and electrochemical impedance spectroscopy (EIS). The microstructures and the corroded surfaces of these anodes were studied by scanning electron microscopy (SEM) and emission spectrum analysis (ESA). The phase structures and the corrosion products of the anodes were analyzed by X-ray diffraction (XRD). The results show that zinc promotes the grain refinement of Mg-6%Al-5%Pb anode and makes the average discharge potential of Mg-6%Al-5%Pb anode more negative during galvanostatic test. Mg-6%Al-5%Pb anode with the addition of 1% (mass fraction) zinc has the best electrochemical performance. The activation mechanism of zinc to Mg-6%Al-5%Pb anode is as follows: The hydrolyzation of dissolved Zn2+ ions reduces the pH value of the solution near the surface of the anode and accelerates the dissolution of Mg(OH)2 film; The precipitated Zn(OH)2 with similar structure as Mg(OH)2 combines with Mg(OH)2 film easily and makes it break down.
Key words:
magnesium anode; alloying; electrochemistry; electrochemical discharge activity;
1 Introduction
Because of the rapid activation, high cell voltage, wide voltage range, high power density capability, relatively low density, low electrode potential and long unactivated storage life [1-4], magnesium alloys have been developed as anode materials used in seawater battery system and cathodic protection such as sonobuoys, beacons, emergency equipments, balloon batteries and life jackets [5-8].
Electrochemical discharge activity is a very important performance for magnesium anode materials used in seawater battery. High system activity always leads to negative discharge potential, short incubation period for activation process and high power density capability. AP65 is one of these magnesium anodes with a nominal composition (mass fraction) of 6% Al, 5% Pb and balance Mg. UDHAYAN and BHATT [9] studied the electrochemical behavior of AP65 in various concentrations of magnesium perchlorate solutions and found that its electrode/electrolyte interfacial process is determined by an activation-controlled reaction. Previous work showed that the activation mechanism of AP65 is dissolving-reprecipitation and there is a synergistic effect between aluminium and lead. The precipitated lead oxides on the surface of the alloy facilitates the precipitation of Al(OH)3, which peels the Mg(OH)2 film in the form of Al(OH)3·2Mg(OH)2 and activates the magnesium matrix [10]. Zinc is another alloying element added into the magnesium auode to improve its electrochemical performance. CAO et al [4] reported the electrochemical oxidation behavior of Mg-Li-Al-Ce-Zn and Mg-Li-Al- Ce-Zn-Mn anodes in NaCl aqueous solution and found that zinc and manganese could improve the electrochemical activity of magnesium anode. However, there are few reports about the electrochemical behavior of Mg-6%Al-5%Pb anode with the addition of zinc and the activation mechanism of zinc to Mg-6%Al-5%Pb anode is not clearly understood. BALASUBRAMANIAN et al [11] studied the electrochemical performance of AZ31 alloy in de-ionized water and 3.3% NaCl solution and found that the activation time is short but the average discharge potential of this alloy is not very negative. This means that lead in Mg-6%Al-5%Pb anode cannot be completely substituted by zinc even though lead is not environmentally friendly. In this work, Mg-6%Al-5%Pb anodes added with different contents of zinc were prepared. Pure magnesium was also included in this work for comparison. The electrochemical behaviors of these anodes in NaCl aqueous solution were studied. The purpose of this work is to get the optimal content of zinc in Mg-6%Al-5%Pb anode to achieve the best electrochemical performance and to study the activation mechanism of zinc to Mg-6%Al-5%Pb anode in 3.5% NaCl aqueous solution.
2 Experimental
Mg-6%Al-5%Pb (mass fraction) anodes with the nominal compositions of 0, 0.2%, 1%, 1.5%,2% Zn were prepared from ingots of pure magnesium (99.99%), pure aluminium (99.99%), pure lead (99.99%) and pure zinc (99.99%) by induction melting at 750 °C with the protection of argon. The molten metal was poured into a stainless steel mold and cooled down to room temperature under argon atmosphere. All these magnesium alloy ingots were solution treated at 400 °C for 24 h and water quenched. The microstructures of the magnesium anode specimens were observed by a Quanta-200 scanning electron microscopy (SEM) after preparation by successive mechanical grinding to 1 200 grit SiC paper, successive polishing to 0.5 μm diamond, washing and drying. The phase structures of these anodes were analyzed by X-ray diffraction (XRD) with a scan rate of 0.6 (°)/min.
The electrochemical measurements were performed by an IM6ex potentiostat with a standard three-electrode glass cell. The electrolyte solution with the volume of 80 mL in the glass cell was made with analytical grade reagents and de-ionized water. Each specimen for the electrochemical measurement was encapsulated in epoxy with a surface area of 10 mm×10 mm exposed to 3.5% NaCl aqueous solution. The specimen surface was ground successively to 1 200 grit SiC paper. A platinum gauze was used as the counter electrode and a saturated calomel electrode (SCE) as the reference electrode. All potentials were referred to the SCE. The galvanostatic discharge behavior was obtained by galvanostatic tests at an anodic current density of 180 mA/cm2 for 600 s. Electrochemical impedance spectra (EIS) were recorded at the open circuit potential with the excitation voltage of 5 mV and the frequency varied from 10 kHz to 0.05 Hz.
After galvanostatic test, the corroded surfaces of the anodes were examined by SEM and emission spectrum analysis (ESA). The phase structures of the corrosion products were analyzed by XRD with a scan rate of 0.6 (°)/min. The pH values of the electrolyte solutions after galvanostatic tests were measured by a PHS-3C pH meter. The concentrations of dissolved Mg2+ and Zn2+ ions after galvanostatic tests were determined by atomic absorption spectrometry.
3 Results and discussion
3.1 Microstructures of Mg-6%Al-5%Pb anodes
Figures 1(a) and (b) show the SEM images of the microstructures of Mg-6%Al-5%Pb and Mg-6%Al- 5%Pb-1%Zn anodes, respectively. The microstructures of other specimens, which are not shown here, are similar to those of Mg-6%Al-5%Pb and Mg-6%Al- 5%Pb-1%Zn anodes. According to Figs. 1(a) and (b), the average equiaxial grain sizes of Mg-6%Al-5%Pb and Mg-6%Al-5%Pb-1%Zn anodes are 180.90 and 62.35 μm, respectively. This means that zinc promotes the grain refinement of Mg-6%Al-5%Pb anode. The microstructures of the two specimens are composed single phase, indicating that the alloying elements are dissolved in the magnesium matrix during solution treatment at 400 °C. The elemental mapping of zinc of Mg-6%Al-5%Pb-1%Zn anode (Fig. 1(c)) shows that zinc distributes homogeneously in the magnesium matrix. The XRD patterns shown in Figs. 2(a) and (b) imply that only α-Mg phase exists in both specimens.
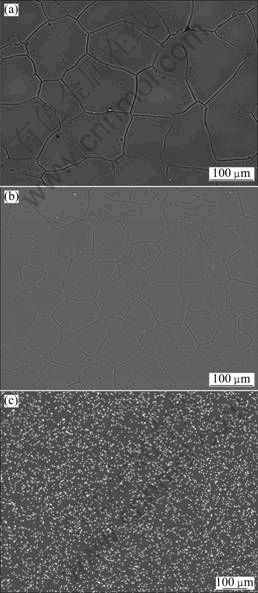
Fig. 1 SEM images of Mg-6%Al-5%Pb anode (a) and Mg-6%Al-5%Pb-1%Zn anode (b) and elemental mapping of zinc in Mg-6%Al-5%Pb-1%Zn anode (c)
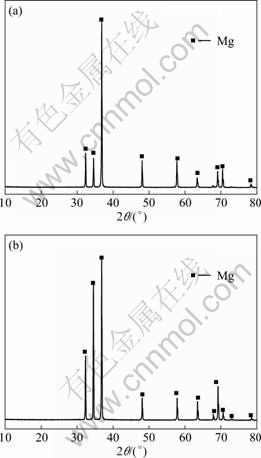
Fig. 2 XRD patterns of Mg-6%Al-5%Pb (a) and Mg-6%Al- 5%Pb-1%Zn (b) anodes
3.2 Influence of zinc content on potential-time curves of Mg-6%Al-5%Pb anodes
Figure 3 shows the galvanostatic discharge behavior of pure magnesium and Mg-6%Al-5%Pb anodes with different contents of zinc at the anodic current density of 180 mA/cm2. All these discharge potential curves were measured by galvanostatic tests immediately after the specimens were immersed in 3.5% (mass fraction) NaCl solution at room temperature. These curves were used to measure the average discharge potentials of magnesium anodes at a certain anodic current density. The specimen with more negative potential always has stronger electrochemical discharge activity and better performance. According to Fig. 3, the potential of pure magnesium increases during the whole process of galvanostatic test (600 s). This indicates that the corrosion products cannot be removed easily via self-peeling and the potential polarizes severely. At the onset of discharge potential-time curves of Mg-6%Al-5%Pb anodes with different contents of zinc, there is an incubation period during which the potential increases rapidly to the maximum value, indicating that an oxide film forms on the surface of the specimen. The subsequent decrease of the potential indicates the breakdown of the oxide film with the effect of Cl- ions in the electrolyte solution [11-16]. After the oxide film is broken down, the potential comes into steady state and the dynamic balance between the advance of the corrosion and self-peeling of the corrosion products is established. The curves of Mg-6%Al-5%Pb anodes with different contents of zinc are even, showing that the corrosion products are removed easily via self-peeling from the surfaces of the specimens.
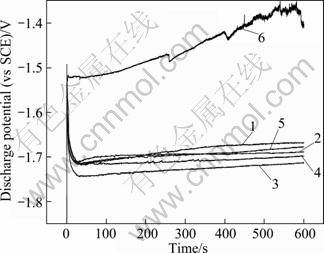
Fig. 3 Galvanostatic discharge behavior of pure magnesium and Mg-6%Al- 5%Pb anodes at 180 mA/cm2 in 3.5% NaCl solution at room temperature: 1—Mg-6%Al-5%Pb; 2—Mg-6%Al- 5%Pb-0.2%Zn; 3—Mg-6%Al-5%Pb-1%Zn; 4—Mg-6%Al- 5%Pb-1.5%Zn; 5—Mg-6%Al-5%Pb-2%Zn; 6—Pure Mg
3.3 Influence of zinc content on average discharge potential and concentration of dissolved Mg2+ ions of Mg-6%Al -5%Pb anodes
Figure 4 shows the average discharge potential and concentration of dissolved Mg2+ ions as a function of content of zinc in Mg-6%Al-5%Pb anode after galvanostatic tests at 180 mA/cm2 for 600 s in 3.5% NaCl solution. The average potentials of Mg-6%Al- 5%Pb anodes with different contents of zinc were calculated by the corresponding discharge potential-time curves shown in Fig. 3. The concentration of dissolved Mg2+ ions was determined by atomic absorption spectrometry. It can be seen from Fig. 4 that the average discharge potential of Mg-6%Al-5%Pb anode decreases with increasing the content of zinc until 1%. Thereafter, the potential increases with the increase of content of zinc. The average potential of Mg-6%Al-5%Pb anode is the most negative (-1.726 V) with the addition of 1% zinc. Besides, the average potentials of Mg-6%Al-5%Pb anodes added with zinc are more negative than those of Mg-6%Al-5%Pb anode without the addition of zinc (-1.686 V), indicating that zinc makes the discharge potential of Mg-6%Al-5%Pb anode more negative. The concentration of dissolved Mg2+ ions increases with increasing the content of zinc in the magnesium matrix until 1%. Thereafter, the concentration of Mg2+ ions decreases with the increase of zinc content. This means that the more negative discharge potentials of Mg-6%Al-5%Pb anodes are always associated with the higher concentration of dissolved Mg2+ ions and the stronger electrochemical activity. With the addition of 1% zinc, the discharge activity of Mg-6%Al-5%Pb is the strongest and it has the best electrochemical performance.
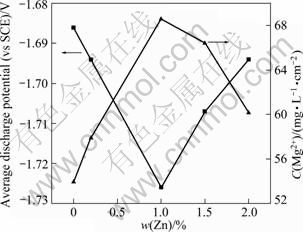
Fig. 4 Average discharge potential and concentration of dissolved Mg2+ ions as function of zinc content in Mg-6%Al-5%Pb anode after galvanostatic tests at 180 mA/cm2 for 600 s in 3.5% NaCl solution
3.4 Influence of zinc content on dissolved Zn2+ ions and pH values of electrolyte solutions after galvanostatic tests
Figure 5 shows the concentration of dissolved Zn2+ ions and pH value of the electrolyte solution as a function of zinc content in Mg-6%Al-5%Pb anode after galvanostatic tests at 180 mA/cm2 for 600 s in 3.5% NaCl solution. The concentration of Zn2+ ions increases with increasing the content of zinc in Mg-6%Al-5%Pb anode until 1%. Thereafter, the concentration of Zn2+ ions decreases with the increase of content of zinc. The concentration of dissolved Zn2+ ions is the highest (2.26 mg/(L·cm2)) with 1% zinc in the magnesium matrix of Mg-6%Al-5%Pb anode. The pH value of the electrolyte solution after galvanostatic test decreases with increasing the content of zinc in Mg-6%Al-5%Pb anode until 1%. Thereafter, the pH value increases with the increase of content of zinc. This means that the lower pH value of the electrolyte solution after galvanostatic test is always associated with the higher concentration of dissolved Zn2+ ions. The hydrolyzation of dissolved Zn2+ ions reduces the pH value of the electrolyte solution during galvanostatic test.
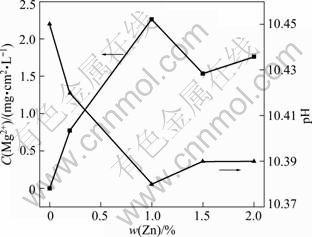
Fig. 5 Concentration of dissolved Zn2+ ions and pH value of electrolyte solution as function of zinc content in Mg-6%Al-5%Pb anode after galvanostatic tests at 180 mA/cm2 for 600 s in 3.5% NaCl solution
3.5 Corroded surfaces and corrosion products of Mg-6%Al-5%Pb and Mg-6%Al-5%Pb-1%Zn anodes after galvanostatic tests
Figures 6(a) and (b) show the SEM image and corresponding ESA results of the corroded surface of Mg-6%Al-5%Pb anode after galvanostatic test, respectively. It can be seen from Fig. 6(a) that the surface of Mg-6%Al-5%Pb anode is covered with thick corrosion products which have wide circular cavity with many cracks. According to the ESA results shown in Fig. 6(b), the content of aluminium on the corroded surface of Mg-6%Al-5%Pb anode is 2.51% (mass fraction) while that of lead is 2.97% (mass fraction), which are lower than the nominal compositions of aluminium and lead in the magnesium matrix of Mg-6%Al-5%Pb anode, indicating that aluminium and lead are removed with the corrosion products from the surface of anode during galvanostatic test. The existence of aluminium and lead on the corroded surface is attributed to the reprecipitation of dissolved Al3+ and Pb2+ ions during galvanostatic test. Figure 6(c) shows the XRD pattern of the corrosion products of Mg-6%Al-5%Pb anode after galvanostatic test. These corrosion products are removed from the surface of anode via self-peeling during galvanostatic test and precipitate on the bottom of the glass cell. They are collected by suction filtration and analyzed by XRD diffractometry. According to Fig. 6(c), the corrosion products are mainly Mg(OH)2 and the Mg(OH)2 film forms by a precipitation reaction when the Mg2+ ion concentration at the corroding surface exceeds the solubility limit [13]. Besides, Al(OH)3, Al(OH)3·2Mg(OH)2 and Pb2O3 are also detected in the corrosion products. Previous work showed that the activation mechanism of Mg-6%Al-5%Pb anode is dissolving-reprecipitation and there is a synergistic effect between aluminium and lead. The precipitated lead oxides on the surface of the anode facilitate the precipitation of Al(OH)3, which peels off the Mg(OH)2 film in the form of Al(OH)3·2Mg(OH)2 and activate the magnesium matrix [10].
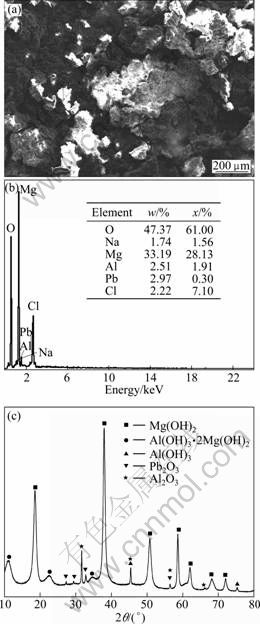
Fig. 6 SEM image (a), corresponding ESA spectrum (b) of corroded surface of Mg-6%-5% Pb anode and XRD pattern of corrosion products (c) removed from surface of Mg-6%-5%Pb anode via self-peeling after galvanostatic test in 3.5% NaCl solution
Figures 7(a) and (b) show the SEM image and corresponding ESA results of the corroded surface of Mg-6%Al-5%Pb-1%Zn anode after galvanostatic test, respectively. It can be seen from Fig. 7(a) that the corrosion products on the surface of Mg-6%Al- 5%Pb-1%Zn anode are thinner and have more cracks than those of Mg-6%Al-5%Pb anode, thus they can be removed from the surface of the anode easily via self-peeling and the activation can be improved. According to the ESA results shown in Fig. 7(b), the content of aluminium on the corroded surface of Mg-6%Al-5%Pb-1%Zn anode is 0.68% (mass fraction) while that of lead is 0.58% (mass fraction), which are lower than those of Mg-6%Al-5%Pb anode shown in Fig. 6(b). The content of zinc on the corroded surface is 0.80% (mass fraction), which is almost the same as the nominal composition of zinc in the magnesium matrix of Mg-6%Al-5%Pb-1%Zn anode. The existence of zinc on the corroded surface is attributed to the reprecipitation of dissolved Zn2+ ions during galvanostatic test as those of aluminium and lead. Therefore, it can be concluded that zinc makes the corrosion products break down easily during galvanostatic test, leading to the low content of aluminium and lead on the corroded surface. Figure 7(c) shows the XRD pattern of the corrosion products of Mg-6%Al-5%Pb-1%Zn anode after galvanostatic test. These corrosion products are removed from the surface of anode via self-peeling during galvanostatic test and are collected by suction filtration. According to the XRD pattern, the corrosion products of Mg-6%Al-5%Pb- 1%Zn anode are similar to those of Mg-6%Al-5%Pb anode. The difference is that Zn(OH)2 exists in the corrosion products of Mg-6%Al-5%Pb-1%Zn anode. This means that the dissolved Zn2+ ions are precipitated in the form of Zn(OH)2 during galvanostatic test and are removed with Mg(OH)2 film. According to Fig. 7(c), the peaks of Zn(OH)2 are almost consistent with those of Mg(OH)2. This means that they have similar structures, which is verified by the space groups and lattice constants of Mg(OH)2 and Zn(OH)2 obtained from PDF card, as listed in Table 1. With similar structures, Mg(OH)2 and Zn(OH)2 have good compatibility and it is easy for Zn(OH)2 to combine with Mg(OH)2. As a result, the breakdown of Mg(OH)2 film can be accelerated, leading to the strong discharge activity of Mg-6%Al-5%Pb- 1%Zn anode.
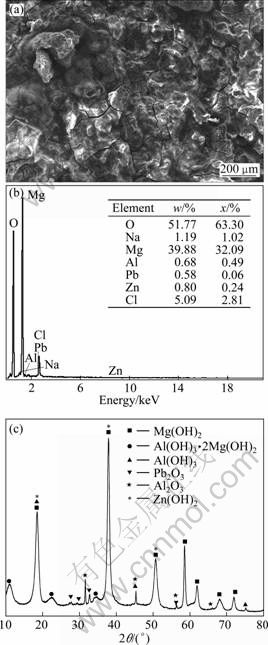
Fig. 7 SEM image (a), corresponding ESA spectrum (b) of corroded surface of Mg-6%Al-5%Pb-1%Zn anode and XRD pattern of corrosion products (c) removed from surface of Mg-6%Al-5%Pb-1%Zn anode via self-peeling after galvanostatic test in 3.5% NaCl solution
3.6 Electrochemical impedance spectra (EIS) of Mg-6%Al-5%Pb and Mg-6%Al-5%Pb-1%Zn anodes
Figure 8 shows the electrochemical impedance spectra (EIS) of Mg-6%Al-5%Pb and Mg-6%Al-5%Pb- 1%Zn anodes. The EIS of other Mg-6%Al-5%Pb anodes have similar shapes as those of Mg-6%Al-5%Pb and Mg-6%Al-5%Pb-1%Zn anodes. This means that the electrochemical mechanisms of Mg-6%Al-5%Pb anodes with different contents of zinc are similar in 3.5% NaCl solution. According to the Nyquist diagram (Fig. 8(a)), there are two capacitive loops for both Mg-6%Al-5%Pb and Mg-6%Al-5%Pb-1%Zn anodes. The capacitive loop at high frequency range is equivalent to a double layer capacitance Cdl and charge transfer resistance Rt. The capacitive loop at low frequency region is related to the corrosion products, Mg(OH)2 film, precipitated on the anode surface. According to CAO [17], if the electrode reaction is controlled by one surface state variable, such as the area fraction (θ) of the anode surface covered with Mg(OH)2 film, then the Faradaic admittance YF can be expressed as
![]() (1)
(1)
where Rt is charge transfer resistance; JF is Faradaic current density; θ is the area fraction of the anode surface covered with Mg(OH)2 film; φ is the potential; ω is the excitation frequency; j=![]() ; θ′=dθ/dt; t is the time.
; θ′=dθ/dt; t is the time.
During Faradaic electrochemical reaction process, Faradaic current density (JF) decreases with the increase of the area fraction (θ) covered with Mg(OH)2 film, thus ![]() . As the potential (φ) increases, more Mg2+ ions dissolve into the solution near the surface of anode, accelerating the precipitation of Mg(OH)2 film on the surface, therefore
. As the potential (φ) increases, more Mg2+ ions dissolve into the solution near the surface of anode, accelerating the precipitation of Mg(OH)2 film on the surface, therefore ![]() . This means
. This means ![]()
![]() in Eq. (1) and there should be a capacitive loop at low frequency range caused by the precipitated Mg(OH)2 film [17], which is consistent with Nyquist diagram shown in Fig. 8(a). The charge transfer resistance Rt can be measured by the diameter of the capacitive loop at high frequency region while the Mg(OH)2 film resistance Rf can be measured by the diameter of the capacitive loop at low frequency range. According to Fig. 8(a), both Rt and Rf of Mg-6%Al- 5%Pb anode are larger than those of Mg-6%Al-5%Pb- 1%Zn anode. According to Bode diagram shown in Fig. 8(b), the total resistance |Z| of Mg-6%Al-5%Pb anode is larger than that of Mg-6%Al-5%Pb-1%Zn anode in the whole range of frequency. The fitted electrochemical parameters for EIS of Mg-6%Al-5%Pb and Mg-6%Al-5%Pb-1%Zn anodes are summarized in Table 2. The equivalent circuits of EIS for both anodes are shown in Fig. 9. Rs is the solution resistance and Cf is the Mg(OH)2 film capacitance. So, it can be concluded that zinc in the magnesium matrix of Mg-6%Al-5%Pb anode can improve its electrochemical discharge activity and accelerate the breakdown of Mg(OH)2 film, leading to the lower values of charge transfer resistance Rt and Mg(OH)2 film resistance Rf of Mg-6%Al-5%Pb-1%Zn anode compared with those of Mg-6%Al-5%Pb anode.
in Eq. (1) and there should be a capacitive loop at low frequency range caused by the precipitated Mg(OH)2 film [17], which is consistent with Nyquist diagram shown in Fig. 8(a). The charge transfer resistance Rt can be measured by the diameter of the capacitive loop at high frequency region while the Mg(OH)2 film resistance Rf can be measured by the diameter of the capacitive loop at low frequency range. According to Fig. 8(a), both Rt and Rf of Mg-6%Al- 5%Pb anode are larger than those of Mg-6%Al-5%Pb- 1%Zn anode. According to Bode diagram shown in Fig. 8(b), the total resistance |Z| of Mg-6%Al-5%Pb anode is larger than that of Mg-6%Al-5%Pb-1%Zn anode in the whole range of frequency. The fitted electrochemical parameters for EIS of Mg-6%Al-5%Pb and Mg-6%Al-5%Pb-1%Zn anodes are summarized in Table 2. The equivalent circuits of EIS for both anodes are shown in Fig. 9. Rs is the solution resistance and Cf is the Mg(OH)2 film capacitance. So, it can be concluded that zinc in the magnesium matrix of Mg-6%Al-5%Pb anode can improve its electrochemical discharge activity and accelerate the breakdown of Mg(OH)2 film, leading to the lower values of charge transfer resistance Rt and Mg(OH)2 film resistance Rf of Mg-6%Al-5%Pb-1%Zn anode compared with those of Mg-6%Al-5%Pb anode.
Table 1 Space groups and lattice constants of Mg(OH)2 and Zn(OH)2 obtained from PDF card
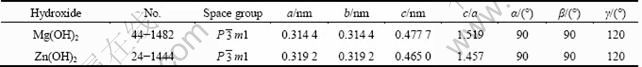
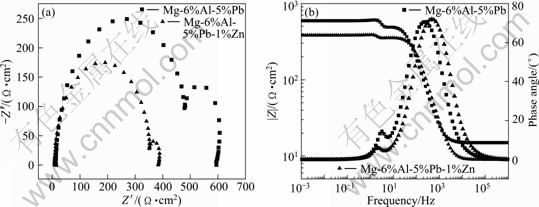
Fig. 8 EIS of Mg-6%Al-5%Pb and Mg-6%Al-5%Pb-1%Zn anodes: (a) Nyquist diagram; (b) Bode diagram
Table 2 Electrochemical parameters obtained by fitting analysis of EIS of Mg-6%Al-5%Pb and Mg-6%Al-5%Pb- 1%Zn anodes

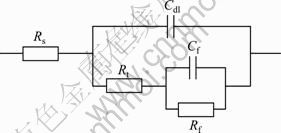
Fig. 9 Equivalent circuits for EIS of Mg-6%Al-5%Pb and Mg-6%Al-5%Pb-1%Zn anodes
3.7 Activation mechanism of zinc to Mg-6%Al-5%Pb anode
The electrochemical reaction equations of Mg-6%Al-5%Pb anode with zinc in the magnesium matrix can be written as follows:
Mg(Al,Pb,Zn)→Mg2++Al3++Pb2++Zn2++9e (2)
2H2O+2e→H2+2OH- (3)
Mg2++2OH-→Mg(OH)2 (4)
Al3++3OH-→Al(OH)3 (5)
4Pb2++O2+8OH-→2Pb2O3+4H2O (6)
Zn2++2H2O→Zn(OH)2+2H+ (7)
The anodic reaction is the dissolution of magnesium and alloying elements while the cathodic reaction is hydrogen evolution, which produces OH- ion and increases the pH value of the electrolyte solution. As the concentration of dissolved Mg2+ ions on the corroding surface exceeds the solubility limit, the precipitation of Mg2+ ions forms a thick Mg(OH)2 film on the surface of magnesium anode. The stability of the Mg(OH)2 film decreases with the decrease of pH value of the solution [13]. The hydrolyzation of dissolved Zn2+ ions decreases the pH value of the solution and makes the Mg(OH)2 film instable, accelerating the dissolution of magnesium matrix. Besides, the dissolved Zn2+ ions are precipitated in the form of Zn(OH)2, which has similar structure as that of Mg(OH)2 film. This means that Mg(OH)2 and Zn(OH)2 have good compatibility and it is easy for Zn(OH)2 to combine with Mg(OH)2. Consequently, the breakdown of Mg(OH)2 film can be accelerated, leading to the stronger discharge activity of Mg-6%Al-5%Pb- 1%Zn anode compared with that of Mg-6%Al-5%Pb anode.
4 Conclusions
1) Zinc promotes the grain refinement of Mg-6%Al-5%Pb anode and makes the average discharge potential of Mg-6%Al-5%Pb anode more negative during galvanostatic test. Mg-6%Al-5%Pb anode added with 1% zinc has the most negative discharge potential (-1.726 V) and best electrochemical performance.
2) The activation mechanism of zinc to Mg-6%Al- 5%Pb anode is summarized as follows: The hydrolyzation of dissolved Zn2+ ions reduces the pH value of the solution near the surface of the anode and accelerates the dissolution of Mg(OH)2 film; The precipitated Zn(OH)2 with similar structure as that of Mg(OH)2 combines with Mg(OH)2 film easily and makes it break down, leading to the stronger discharge activity of Mg-6%Al-5%Pb-1%Zn anode compared with that of Mg-6%Al-5%Pb anode.
References
[1] RENUKA R. Influence of allotropic modifications of surphur on the cell voltage in Mg-CuI(S) seawater activated batter [J]. Materials Chemistry and Physics, 1999, 59(1): 42-48.
[2] FENG Yan, WANG Ri-chu, YU Kun, PENG Chao-qun, LI Wen-xian. Influence of Ga and Hg on microstructure and electrochemical corrosion behavior of Mg alloy anode materials [J]. Transactions of Nonferrous Metals Society of China, 2007, 17: 1363-1366.
[3] RENUK A R. AgCl and Ag2S as additives to CuI in Mg-CuI seawater activated batteries [J]. Journal of Applied Electrochemistry, 1997, 27(12): 1394-1397.
[4] CAO Dian-xue, WU Lin, WANG Gui-ling, LU Yan-zhou. Electrochemical oxidation behavior of Mg-Li-Al-Ce-Zn and Mg-Li-Al-Ce-Zn-Mn in sodium chloride solution [J]. Journal of Power Sources, 2008, 183: 799-804.
[5] FENG Yan, WANG Ri-chu, PENG Chao-qun, WANG Nai-guang. Influence of Mg21Ga5Hg3 compound on electrochemical properties of Mg-5%Hg-5%Ga alloy [J]. Transactions of Nonferrous Metals Society of China, 2009, 19: 154-159.
[6] FIRA S S, KIBL L, LIW L W. Water-activated disposable and long shelf-life microbatteries [J]. Sensors and Actuators A, 2004, 111: 79-86.
[7] VENKATESARA R K. Performance evaluation of Mg-AgCl batteries for under water propulsion [J]. Defense Science Journal, 2001, 5(2): 161-170.
[8] FIDEL G M, JUAN M F, RUBEN D R, GENESCA J. Electrochemical study on magnesium anodes in NaCl and CaSO4-Mg(OH)2 aqueous solutions [J]. Electrochimic Acta, 2006, 51: 1820-1830.
[9] UDHAYAN R, BHATT D P. On the corrosion behavior of magnesium and its alloys using electrochemical techniques [J]. Journal of Power Sources, 1996, 63: 103-107.
[10] WANG Nai-guang, WANG Ri-chu, PENG Chao-qun, FENG Yan, ZHANG Xiang-yu. Influence of aluminium and lead on activation of magnesium as anode [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1403-1411.
[11] BALASUBRAMANIAN R, VELUCHAMY A, VENKATAKRISHNAN N, GANGADHARAN R. Electrochemical characterization of magnesium/silver chloride battery [J]. Journal of Power Sources, 1995, 56: 197-199.
[12] TAMAR Y, MANDLER D. Corrosion inhibition of magnesium by combined zirconia silica sol-gel films [J]. Electrochimica Acta, 2008, 53: 5118-5127.
[13] ZHAO Ming-chun, LIU Ming, SONG Guang-ling, ATRENS A. Influence of pH and chloride ion concentration on the corrosion of Mg alloy ZE41 [J]. Corrosion Science, 2008, 50: 3168-3178.
[14] HIROMOTO S, YAMAMOTO A, MARUYAMA N, SOMEKAWA H, MUKAI T. Influence of pH and flow on the polarization behaviour of pure magnesium in borate buffer solutions [J]. Corrosion Science, 2008, 50: 3561-3568.
[15] ZHAO Ming-chun, SCHMUTZ P, BRUNNER S, LIU Ming, SONG Guang-ling, ATRENS A. An exploratory study of the corrosion of Mg alloys during interrupted salt spray testing [J]. Corrosion Science, 2009, 51:1277-1292.
[16] SONG Y W, SHAN D Y, HAN E H. Corrosion behaviors of electroless plating Ni-P coatings deposited on magnesium alloys in artificial sweat solution [J]. Electrochimica Acta, 2007, 53: 2009-2015.
[17] CAO Chu-nan. Principles of electrochemistry of corrosion [M]. Beijing: Chemical Industry Press, 2008: 76-77. (in Chinese)
(Edited by YANG Bing)
Foundation item: Project(JPPT-115-168) supported by the National Key Science and Technological Program of China
Received date: 2010-12-27; Accepted date: 2011-05-21
Corresponding author: WANG Ri-chu, Professor, PhD; Tel: +86-731-88836638; E-mail: wrc@csu.edu.cn
Abstract: Mg-6%Al-5%Pb (mass fraction) anodes with different contents of zinc were prepared by melting and casting. The electrochemical discharge behavior of these anodes in 3.5% NaCl solutions was investigated by galvanostatic test and electrochemical impedance spectroscopy (EIS). The microstructures and the corroded surfaces of these anodes were studied by scanning electron microscopy (SEM) and emission spectrum analysis (ESA). The phase structures and the corrosion products of the anodes were analyzed by X-ray diffraction (XRD). The results show that zinc promotes the grain refinement of Mg-6%Al-5%Pb anode and makes the average discharge potential of Mg-6%Al-5%Pb anode more negative during galvanostatic test. Mg-6%Al-5%Pb anode with the addition of 1% (mass fraction) zinc has the best electrochemical performance. The activation mechanism of zinc to Mg-6%Al-5%Pb anode is as follows: The hydrolyzation of dissolved Zn2+ ions reduces the pH value of the solution near the surface of the anode and accelerates the dissolution of Mg(OH)2 film; The precipitated Zn(OH)2 with similar structure as Mg(OH)2 combines with Mg(OH)2 film easily and makes it break down.


