
层状双金属氢氧化物催化生物质热解制高品质合成气应用前景展望
赵文祥,杨双霞,陈雷,孙来芝,谢新苹,伊晓路,司洪宇,赵保峰,华栋梁
(齐鲁工业大学(山东省科学院) 能源研究所 山东省生物质气化技术重点实验室,山东 济南,250014)
摘 要:
物质基液体燃料和高附加值化学品的重要原料,热解是实现生物质在低温下向高品质合成气转化的有效途径之一。在热解过程中,催化剂能够促进生物质热解挥发分中焦油大分子的裂解、重整转化,降低CO2和CH4等气体组分体积分数,同时通过水汽变换反应调整气体组分,最终实现合成气的净化和调变,而高活性、热稳定和抗积碳催化剂的设计研发始终是生物质热转化过程的研究热点和难点。针对生物质热转化过程,本文综述了催化剂活性组分调变、载体组成结构修饰和物化特性优化3个方面最新研究进展。针对催化剂在反应过程中易高温烧结、积碳失活问题,结合层状双金属氢氧化物(LDHs,水滑石)材料独特的层板元素比例可调、金属阳离子呈原子水平分散、物化结构特性以及其作为催化剂前体/载体在多相催化领域的优异性能,展望了LDHs材料在生物质催化热解制高品质合成气的应用前景。以LDHs作为催化剂前体,经拓扑结构转变制备的金属/金属氧化物复合材料(M/MMO)可实现活性组分的高度分散和酸碱性调控,进而有效解决热转化过程中催化剂高温烧结问题,并抑制积碳形成,促进生物质向高品质合成气的高效、稳定转化。
关键词:
中图分类号:TK6 文献标志码:A 开放科学(资源服务)标识码(OSID)
文章编号:1672-7207(2021)06-2040-12
Prospect of layered double hydroxide for high quality syngas production from catalytic pyrolysis of biomass
ZHAO Wenxiang, YANG Shuangxia, CHEN Lei, SUN Laizhi, XIE Xinping, YI Xiaolu, SI Hongyu, ZHAO Baofeng, HUA Dongliang
(Shandong Provincial Key Laboratory of Biomass Gasification Technology, Energy Research Institute, Qilu University of Technology (Shandong Academy of Sciences), Jinan 250014, China)
Abstract: Syngas was an important raw material for producing liquid fuels and a variety of high value-added products. Pyrolysis technology was one of the effective ways to produce synthesis high quality syngas from biomass at low temperature. During pyrolysis progress, tar macromolecules in pyrolytic volatiles can be effectively decomposed and reformed by catalyst. At the same time, the volume fraction of CO2 and CH4 can decrease and the gas components can be modulated via water gas shift reaction, resulting in producing high quality syngas. However, design and development of catalysts with high activity, thermal stability and suppression of coke deposition have been the hotspots and difficulties. In this paper, the latest research progress in terms of active composition modulation, structure and physicochemical properties optimization of support during catalytic pyrolysis of biomass were comprehensively reviewed. Due to the tunability of metal component and content in layers, high dispersion of metal cations on an atomic level and unique basic properties, LDHs materials show excellent performances in heterogeneous catalysis. Therefore, their potential application in biomass catalytic pyrolysis for syngas production was prospected here. The mixed metal and metal oxides(M/MMO) derived from LDHs precursors via topotactic transformation exhibit highly dispersed active component and tunable acid-base properties, which can effectively resolve the problem of sintering at high temperature and formation of coke. As a result, biomass can be converted into high quality syngas efficiently and stably.
Key words: biomass; thermal conversion; syngas; catalyst; layered double hydroxides
当前,传统化石能源(石油、煤和天然气)过度消耗并释放出大量CO2和SO2等气体,导致了全球变暖、酸雨和气候变化等严重的环境问题[1-2]。开发可再生、生态友好型清洁能源,可持续地补充或替代化石燃料,对人类健康和生态环境都至关重要[3]。生物质作为一种来源广泛、可再生和碳零排放的代表性清洁能源受到众多学者的关注。
近几十年来,热解技术在生物质转化方面得到了广泛研究。生物质经过热解得到的气体产物主要包括H2,CO,CO2,CH4和一些小分子烃类。其中由CO和H2组成的合成气可以直接用作气体燃料,也可以作为原料气体通过费托合成技术将其转化成液体燃料和高值化学品,是一种极具应用前途的化工原料。在没有催化剂的条件下,热解气产率有限,而且在热解过程中伴随着产生大量焦油,会严重影响合成气的品质并造成相关设备管道堵塞和腐蚀[4]。此外,由于生物质低氢碳比、高氧碳比的结构特点,其直接热解所得合成气的氢碳比通常较低,难以满足液体燃料合成过程,需经水煤气变换过程将氢气与一氧化碳物质的量之比调控至2~3或者更高。因此,促进焦油的高效转化和提高气体产物中H2的选择性是实现合成气高效再利用的关键[5],也是生物质热解过程中需要重点关注和解决的问题。
在热解过程中加入适宜催化剂,可将焦油催化裂解和重整为小分子气体产物,同时,通过气体组分相互转化调整气体产物分布,进而减少热解气中焦油含量并提升合成气的品质。然而,催化剂在使用过程中往往面临高温烧结和积碳沉积问题,导致其催化活性迅速降低甚至失活,调控优化催化剂组成、结构和物化特性,实现活性位点高度分散并充分暴露,同时促进反应物的扩散传质过程,对实现生物质向高品质合成气的高效、稳定转化是非常有意义的。
1 催化剂性能优化研究现状
1.1 活性组分调变
生物质热解过程中,单金属催化剂通常存在催化活性低或易积碳失活的问题。在催化剂中引入活性金属助剂,构建双金属或多金属催化剂,能够提高催化剂活性和抗积碳能力。ZHANG等[6]在松木屑和废塑料的催化热解实验中比较了双金属催化剂Ni-Fe@CNF/PCs和单金属催化剂Ni@CNF/PCs、Fe@CNF/PCs的催化性能,发现在生物质与塑料质量比为1、催化温度为700 ℃的条件下,Ni-Fe@CNF/PCs双金属催化剂具有更高焦油转化率(87.9%),H2和CO产量(分别为24.73 mmol/g和6.79 mmol/g)和H2与CO物质的量比(3.64)。这主要是Fe0.64Ni0.36合金能够协同发挥Ni和Fe双活性位催化作用,促进焦油裂解、重整以及水煤气变换反应。此外,多孔碳纳米纤维载体显著增加了催化剂的比表面积和孔体积,进一步提高了催化剂的活性和抗积碳能力。
JIN等[7]研究了Ni-Mg-Al催化体系Ca的引入对反应性能的影响。在木屑热解-水蒸气重整实验中,Ni-Ca-Mg-Al催化剂有更好的H2选择性和较低的CH4生成量。当Ca的负载量为0.5%时,气体产量达到最大(74.4%,质量分数),气体中H2和CO体积分数分别达到49.0%和22.3%,CH4体积分数仅为7.7%。这主要归因于Ca的引入一方面增加了活性位点数量,促进焦油的催化裂解,另一方面通过对CO2原位吸收,强化了水汽变换反应,从而促进H2的生成。随着Ca添加量增加,NiO相增加,当Ca添加量为0.5%时,催化剂中NiO小颗粒比例最高,占比为54.9%。
KUMAGAI等[8]研究了Ca添加量对Ni-Ca-Mg-Al催化剂在生物质/塑料热解制合成气过程催化活性的影响,发现当Ni,Ca,Mg和Al物质的量比为1:1:1:4时,H2产量最高,达到39.6 mol/g,H2与CO物质的量比为1.9。因此,在催化剂制备过程中,添加适量的活性金属助剂不仅能够提高活性组分的分散度,而且能提供更多的活性位点,有效提高催化反应活性。
1.2 载体组成结构修饰
在载体选择方面,以纳米多孔材料为载体的金属催化剂往往具有明确结构特征、较高比表面积和较小金属颗粒粒径[9]。载体本身的空间构型使催化剂具备一定形状,从而提高活性位点的分散度以及有效利用率,而适宜的孔结构则可提高催化剂的热稳定性,使孔内金属活性位更易与挥发分中大分子化合物充分接触,提升催化反应活性。
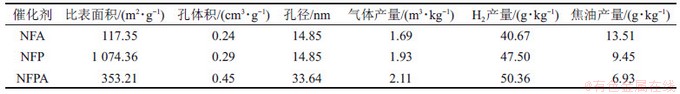
SHI等[10]以粉状活性炭(PAC)和γ-Al2O3为载体,制备了NiO-Fe2O3/PAC-γ-Al2O3(NFPA),NiO-Fe2O3/PAC(NFP)和NiO-Fe2O3/γ-Al2O3(NFA)催化剂,对比研究了单一载体催化剂和复合载体催化剂在油菜秸秆热解过程中的催化性能(如表1所示[10]),发现相较于2种单一载体催化剂,复合载体催化剂反应性能显著提高。在NFPA催化剂作用下,产气量为2.11 m3/kg、H2产量为50.36 g/kg、焦油产量为6.93 g/kg,H2与CO物质的量比1.58。这可能归因于NFPA具有较大孔径和孔体积,导致孔中的Ni/Fe活性颗粒能够充分催化焦油裂解生成H2和CO。在稳定性方面,使用过的NFPA催化剂比表面积(362.18 m2/g)变化较小,表现出很强的抗积碳和抗失活能力。
表1 催化剂载体组成对热解产物的影响[10]
Table 1 Effect of catalyst support compositions on product yields during pyrolysis process
CHEN等[11]在Al2O3载体中进一步引入Ca组分,制备出Ni/CaAlx催化剂,并将其用于木屑热解-蒸汽重整实验,发现Ca的引入可以减小活性NiO颗粒粒径,使其高度分散于Ca-Al复合载体中,NiO粒径基本保持在10 nm左右。当Ca与Al物质的量比为3:1时,气体中合成气H2+CO体积分数最高,达90%,H2与CO物质的量比为1.01。随着Ca含量增加,催化剂表面积碳量也随之减少。
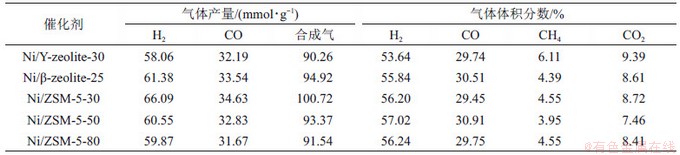
YAO等[12]对比研究了负载在不同分子筛载体上的Ni基催化剂对废塑料热解-蒸汽重整的催化性能(表2)。虽然β-zeolite-25(比表面积为324 m2/g,孔体积为0.657 cm3/g)和Y-zeolite-30(比表面积为500 m2/g,孔体积为0.215 cm3/g)负载Ni基催化剂具有较大的比表面积和孔体积,ZSM-5载体(比表面积为229 m2/g,孔体积为0.135 cm3/g)在H2选择性、合成气产率方面却表现出较高反应活性。经进一步研究发现,随着Si与Al物质的量比增加,中孔数量减少,Ni分散度降低,H2和CO产量呈下降趋势。因此,催化剂的反应活性是载体比表面积、孔体积、孔径以及活性粒子尺寸和分散度协同作用的结果。
表2 在850 ℃和6 g/h水蒸气流量下废塑料热解-蒸汽重整生成的气体组分[12]
Table 2 Gas components by pyrolysis-steam reforming of waste plastics at 850 ℃ and 6 g/h steam flow rates[12]
1.3 载体物化性质优化
催化剂的催化性能与活性金属组成、载体种类和孔结构有关,载体表面酸碱性也是影响焦油大分子裂解转化以及合成气组成调变的重要因素。
YE等[13]在木屑催化热解实验中设计合成了不同酸度的Ni/MCM-41催化剂(酸性、弱酸性和无酸性),发现酸性较强的Ni/H-[Al]MCM-41催化剂对H2的生成有着明显促进作用,其H2产量(21.6 mmol/g)是另外2种催化剂(6.7 mmol/g和9.8 mmol/g)的2~3倍,这主要是由于较多的酸性位点促进了焦油化合物的吸附以及C—C的断裂,进而生成了更多的H2和CO。然而,催化剂酸性中心增多往往会导致严重的积碳行为。
DONG等[14]在木屑热解-蒸汽重整实验中,引入ZnO来降低Ni-Al2O3催化剂的表面酸性。发现ZnO增加了载体碱性位点的数量,抑制了裂解和聚合反应,促进水蒸气和炭的反应,从而抑制催化剂表面碳沉积,当Ni负载量为35%,Zn与Al物质的量比为1/4时,反应后NiZnAlOx催化剂表面积碳量几乎为0,此时气体产率和H2产量分别74.8%(质量分数)和20.1 mmol/g。
LI等[15]则研究了稻草热解蒸汽重整制备合成气过程中MgO对Ni/γ-Al2O3催化剂反应活性的影响,发现1.0%(质量分数)的MgO的加入可以充分暴露Ni活性颗粒,强化焦油裂解、重整以及水煤气变换等反应,进而提高催化活性,使H2产量达到了1 194.6 ml/g,H2与CO的物质的量比例为3.9。因此,引入碱性载体,在一定程度上降低了催化剂表面酸性即可提高活性中心分散性,又可提高催化剂抗积碳能力,解决活性中心覆盖失活问题,最终促进目标产物生成。
综上所述,提高活性组分负载量、降低粒子尺寸、提高活性中心分散性以及调控适宜的催化剂酸碱性均可促进生物质向高品质合成气的定向制备,是提高催化剂反应活性和稳定性的有效手段。
2 层状双金属氢氧化物(LDHs)
2.1 LDHs基本结构
层状双金属氢氧化物,也称为类水滑石化合物,是一种具有二维层状纳米结构的阴离子型层状材料,主要由带正电荷的主体层板和带负电荷的层间阴离子组成[16]。LDHs的主体层板是由M2+和M3+的金属离子构筑的带有正电荷的八面体所组成,层间则被可交换的阴离子和结晶水所占据[17],如图1所示。LDHs通式可表示为[M1-x2+Mx3+(OH)2]x+(An-)x/n·mH2O,其中M2+为二价阳离子(Mg2+,Zn2+,Ca2+,Ni2+和Cu2+等),M3+为三价阳离子(Al3+,Cr3+和Fe3+等),An-为层间阴离子[18]。当二价金属离子在一定的比例范围内被半径相似的三价离子替代时[19],层板上就会产生多余正电荷,层间的阴离子会提供负电荷,使得LDHs呈电中性[20]。
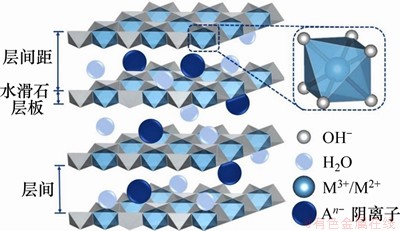
图1 LDHs结构示意图[17]
Fig. 1 Structure diagram of LDHs[17]
2.2 LDHs物化特性
2.2.1 层板金属元素组成及比例的可调控性
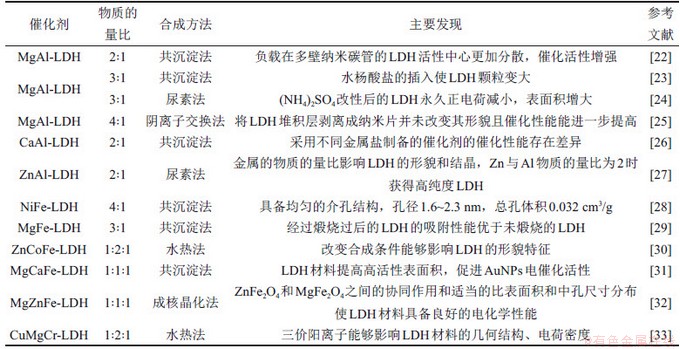
根据实际需要,LDHs的层板化学组成是可调控的[22-33]。层板中M2+和M3+可用其他同价、半径相近的金属阳离子替代,构建一系列应用广泛的二元、三元以及多元的LDHs功能性材料,如表3所示[22-33]。因金属元素自身的性质和金属间的相互作用,不同金属元素组成的LDHs材料的催化特性也存在差异。在合理范围内进一步调整M2+和M3+的物质的量比或相对含量,可以改变层板电荷密度及化学性质等。基于这个特点,研究者可以依照所选定的催化体系,对金属活性组分及含量的组合进行优化。
表3 不同金属元素及比例的二元和三元LDHs材料
Table 3 Binary and ternary LDHs materials with different metal elements and proportions
2.2.2 层板金属元素呈原子水平分散
LDHs层板上金属阳离子呈原子级分散,并在层板上掺杂形成具有大比表面的片层状结构。由于LDHs层板水平方向上的拓扑不变性,以LDHs作为单源前驱体,在高温条件下焙烧得到的金属/金属氧化物复合材料(M/MMO)仍保持金属元素均匀高度分散的结构特征[34],并且金属活性组分与金属氧化物载体之间存在强相互作用,能够有效抑制活性组分的团聚,提高催化剂的热稳定性[35]。
ZHAO等[36]以Fe/Mg/Al-CO32-LDH为前驱体,基于LDHs独特的结构记忆效应,采用焙烧复原的方式制备得到了具有高负载量(1014~1016 m-2)、粒径可控(3~20 nm)、热稳定性强(900 ℃)的高分散负载型Fe纳米复合材料(如图2所示)。将Mo固定在Fe之间可以有效抑制Fe的团聚,进一步提高其分散度和热稳定性,使其在化学气相沉积制备碳纳米管过程中表现出优异的催化活性。
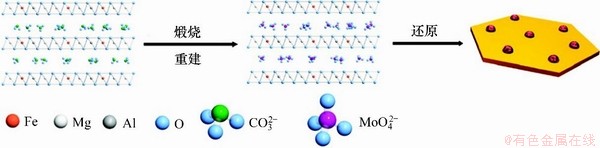
图2 基于Fe/Mg/Al LDH前驱体制备高分散Fe催化材料示意图[36]
Fig. 2 Schematic illustration of fabrication of highly dispersed Fe catalyst from Fe/Mg/Al LDH[36]
YAN等[37]将CuMgMnAl-CO32--LDH为前驱体经焙烧得到的Cu0.5Mg1.5Mn0.5Al0.5Ox复合金属氧化物作为NH3-SCR催化剂,在100~250 ℃低温范围内脱硝效率达到87.0%~96.6%,远高于传统的Mn/γ-Al2O3催化剂的脱硝效率(35.0%~67.2%)。这主要是由于Cu0.5Mg1.5Mn0.5Al0.5Ox活性组分的数量和种类(MnO2和CuO)增加,且纳米颗粒分散度更高,拥有更多的酸位点和更强的还原性。
LIU等[38]对比研究了不同合成路线制备的NiCu/MMO复合金属氧化物在乙炔半加氢反应中的催化性能,发现基于LDHs前体构筑的NiCu/MMO催化剂的反应活性强于传统浸渍法所得NiCu/MMO催化剂。这主要归因于其具有更小颗粒粒径(3.2 nm)和更高的金属分散度(31.4%)。此外,与浸渍法所得金属Ni,Cu和NiCu合金多种物相不同,基于LDHs构筑的NiCu/MMO催化剂具有显著的合金化效应(NiCu合金),大幅度促进了电子从Cu向Ni的转移,而含有丰富电子的Ni更有利于乙烯的吸附,进而提高对乙烯的选择性,同时表现出较强的稳定性和抗结焦能力,如图3所示。
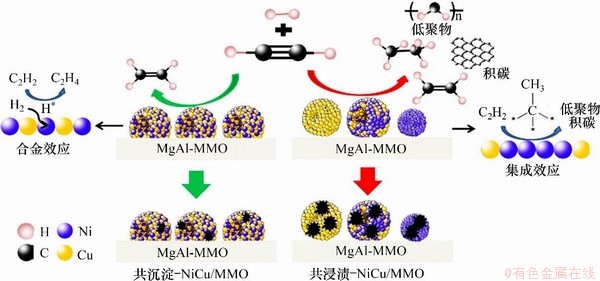
图3 乙炔半加氢双金属催化剂设计思路与反应过程
Fig. 3 Design concept and reaction process of two bimetallic catalysts for semi-hydrogenation of acetylene
2.2.3 焙烧产物MMO表面呈碱性
LDHs材料因为层板带有大量羟基,在通常情况下,LDHs材料及其焙烧生成的MMO均呈现碱性[39]。
RADHA等[40]将Mg2Al-LDH前驱体煅烧得到的MgAl-MMO用于CO2吸附,发现吸附实验后样品表面存在由CO2和MgO生成的单晶碳酸盐,这意味着MgO是主要的碱性位点。
BING等[41]通过焙烧复原方式制备了CaxAl-LDH催化剂,发现CaxAl-LDH内包含1个扭曲的Ca(OH)2八面体结构和1个提供弱Bronsted碱位点的Ca-OH配位,而Bronsted碱位点浓度可通过调节LDH前驱体的Ca与Al物质的量比来增强。优化后的Ca4Al-LDH对异丁醛和甲醛缩合生成羟戊醛具有优异的催化性能,且对羟戊醛的选择性明显高于传统的固体碱催化剂,可以与液体碱相媲美。
GUO等[42]制备了Al2O3负载的Ni5Al-MMO纳米复合材料,并以Ni/Al2O3催化剂作为对比来考察CO2甲烷化性能,发现Ni5Al-MMO催化剂的CO2转化率明显高于Ni/Al2O3的CO2转化率。对比2种催化剂的碱性和氧化还原性能发现,Ni/Al2O3催化剂中只有2个弱碱性位点,而Ni5Al-MMO催化剂同时具备弱碱性位点和中等碱性位点,如图4所示。此外,Ni5Al-MMO更易被H2还原,生成的金属Ni纳米颗粒和碱性位点协同作用增强了其催化活性。
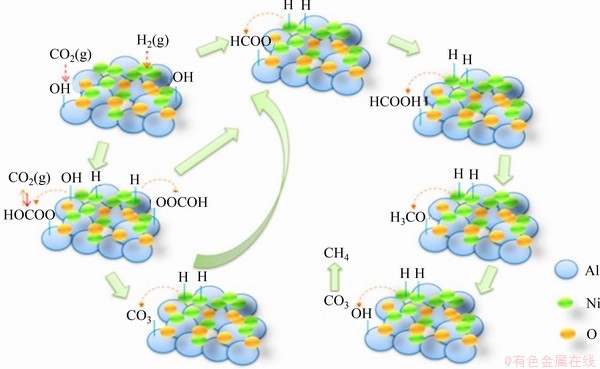
图4 CO2甲烷化的Ni5AL-MO催化剂[42]
Fig. 4 Schematic representation of CO2 methanation by Ni5AL-MO catalyst[42]
2.3 LDHs在多相催化领域中的应用
LDHs独特的结构特征赋予了其一系列独特性质。LDHs的金属离子的可调变性极大丰富其化学性能。基于LDHs材料层板金属阳离子原子水平高度分散的特性,以其为前驱体经层板剥离、晶格限域、插层组装及阵列化等途径可制备得到多孔、高比表面积、活性位高度均匀分布的多相催化剂材料[43],如图5所示。正是因其独特的结构性质,LDHs材料在酯化反应、选择性加氢和电催化等领域得到广泛应用。
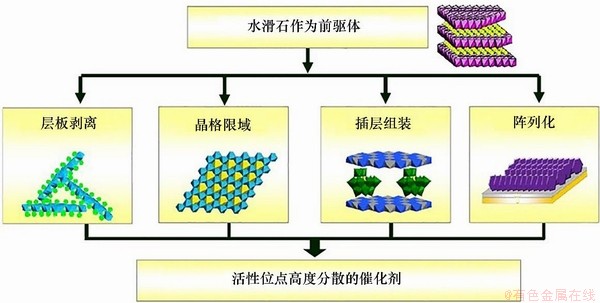
图5 基于层状前驱体制备活性位高分散催化材料的示意图[43]
Fig. 5 Preparation approaches from layered double hydroxide(LDH) materials for catalysts with catalytic sites highly dispersed[43]
2.3.1 酯化反应
LIAO等[44]在甲醇和碳酸丙烯进行酯交换合成碳酸二甲酯反应中,以含有不同碱金属的Ca-M-Al LDH(M=Mg,La,Ce,Y)作为前驱体,制备了一系列Ca-M-Al基复合固体碱催化剂。发现在碱性最强的Ca-Mg-Al催化剂作用下,碳酸丙烯酯的转换率最高,碳酸二甲酯的选择性最高。在稳定性方面,Ca-Mg-Al催化剂在10次循环实验中仍保持最高的催化活性,这主要是由于Ca-Mg-Al催化剂中(Ca+Mg)与Al物质的量比最高,含有更多的不饱和O2-离子,为其提供了最高的碱性位点浓度。
GANDARA-LOE等[45]制备了X-Al-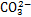
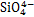
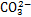
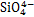
2.3.2 选择性加氢反应
LIN等[46]在肉桂醛选择性加氢反应中使用Mg3Al1-xFex-LDH负载Ir催化剂,研究了不同含量的Fe和Al对催化反应的影响,发现肉桂醇的选择性随着Fe含量增多而升高,但过量添加Fe会降低肉桂醛的转化率。在Mg3Al1-xFex催化剂中,Fe与Ir存在相互作用,Fe含量增多,Fe2+含量也随之增多,正是Fe2+向Ir3+发生电子转移形成了富电子Ir物种和缺电子Fe物种,从而增强了催化剂的催化活性。
TIAN等[47]研究了以CoAl-MMO为载体的Pt/CoAl-MMO复合催化剂在肉桂醛加氢合成肉桂醇反应中的催化性能。相对于Pt/CoAl-LDH催化剂,焙烧后MMO作为载体制备的催化剂表现出更高的催化活性和选择性,且在3次循环内稳定性良好。这可能归因于复合金属氧化物载体增强了金属化合物间的相互作用,从而增强了催化剂的稳定性。
JIN等[48]研究了PdNi/MgAl-MMO催化剂在乙炔加氢制备乙烯反应中的催化活性,在反应温度为70 ℃、体积空速为10 056 h-1、相对压力为0.2 MPa的条件下,乙炔转化率达接近95%,乙烯的选择性达到75%。PdNi/MgAl-MMO较高的活性和稳定性可归因于Pd和Ni间强相互作用改变了催化剂表面H2的吸附/脱附特性,从而减少了-PdH的形成并抑制过度加氢。此外,由于LDH前驱体形成的MgAl-MMO微晶的净捕集效应,为PdNi纳米颗粒提供了固定位置,导致催化剂具有较好的结构稳定性。
2.3.3 电催化反应
CAO等[49]设计合成了一种在石墨烯氧化物(GO)上原位生长的类花状Ni/Fe-LDH三维结构(如图6[49]),并将其用于氧化还原反应(ORR)。发现Ni/Fe-LDH/GO具有较大的正ORR起始点位和较高的阴极电流,在转速为1 600 r/min的0.1 mol/L KOH溶液中,Ni/Fe-LDH/GO的起始还原电位达到0.88 V,电导率约为12.5 S/m,明显高于对照组Ni/Fe-LDH催化剂的电导率。Ni/Fe-LDH/GO催化剂独特的花状结构具有更大比表面积和更多活性位点,有助于ORR反应过程中氧气输送,且加入GO提高了催化剂的电导率并促进电子转移,进而提高了其催化活性和稳定性。
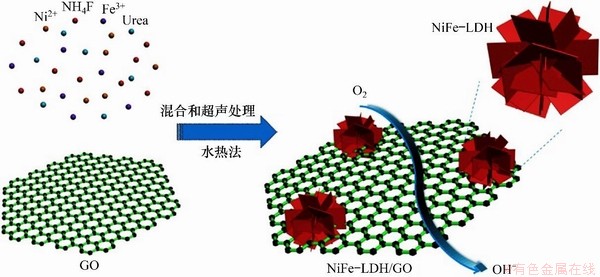
图6 制备Ni/Fe-LDH/GO原理图[49]
Fig. 6 Schematic illustration of preparing NiFe-LDH/GO catalyst[49]
LIANG等[50]将电沉积和水热法相结合合成了具有三维核壳结构的Ni3S2/NiFe-LDH析氧反应(OER)催化剂。发现在1 mol/L KOH溶液中,Ni3S2/NiFe-LDH只需200 mV的电压就能得到10 mA/cm2的电流密度,273 mV的过电压则得到200 mV/cm2的电流密度,而贵金属RuO2催化剂在273 mV的过电压下只能得到9.22 mA/cm2的电流密度。OER性能的增强可归因于Ni3S2/NiFe-LDH催化剂较大的表面积,进而在电催化反应有更多的活性位点,并使气体更容易逸出。
2.4 LDHs在生物质催化转化领域中的应用
合成气作为液体燃料和高附加值化学品的主要原料,其产率和品质是一项不可忽略的问题。近年来,学者们探索了LDHs及其衍生催化剂在生物质热转化领域的应用。
NAVARRO等[51]研究了不同Mg与Al物质的量比例(2,3和4)的Mg-Al-MMO在小麦秸秆快速热解过程的催化性能。发现随着Mg含量增加,Mg-Al-MMO拥有更大孔径、比表面积和更多碱性位点,促进了产气量增加。同时,较高碱性位点浓度促进了醇醛缩合反应和热解产物的酮化作用,降低了生物油的含氧量。
WANG等[52]研究了基于LDH前体构筑的Co/Mg/Al催化剂在生物质水蒸气气化中的催化性能,并与传统的Co/γ-Al2O3催化剂进行对比,发现Co/Mg/Al催化剂具有更高的催化活性和抗积碳能力,H2的生成速率达到1623 mol/min,H的转化率为54%,H2与CO物质的量比为2.3,且焦油含量较低。这主要是由于Co金属颗粒和MgAl2O4固溶体之间具有更强的相互作用,有效抑制了Co金属颗粒的团聚,进而提高了催化活性和抗积碳性能。在此基础上,WANG等[53]进一步考察了Fe的引入对Co/Mg/Al催化性能的影响,发现经合金化后所得Co/Fe/Mg/Al纳米复合材料显示出更强的催化活性和抗积碳性能。
表4 LDHs材料与其他铁基催化剂在催化生物质热解转化合成气的应用
Table 4 Summary of main investigations on syngas production via catalytic biomass conversion
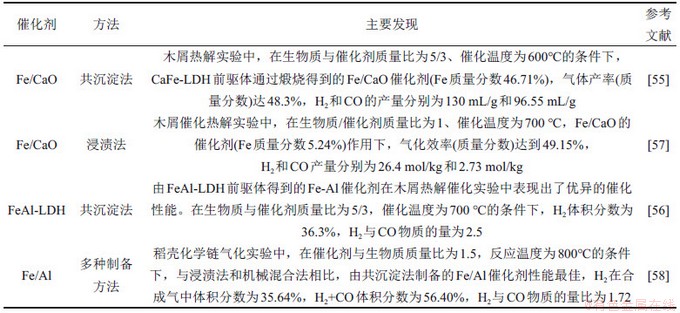
YANG等[54]在催化体系中引入Ca基活性组分,系统研究了LDHs基Fe-Ca催化-吸收双功能催化剂在生物质热解制富氢合成气体系的催化活性和稳定性,其研究成果与其他研究者研究成果对比如表4所示[55-58]。对比传统浸渍法所得Fe-Ca催化剂(H2产量为137 mL/g,H2与CO物质的量比为1.07),基于LDHs前体构筑的Fe-Ca具有更小粒子尺寸、更高CO2吸收性能和更强H2还原特性,热解条件下H2产量达到了152 mL/g,H2与CO物质的量比为1.23。调变CaFe-LDH中Ca与Fe物质的量比例,显著提高了催化剂中活性组分Fe的负载量(46%,质量分数),进一步提高合成气中H2的产率和选择性 [55]。对比不同载体负载Fe基催化剂(Fe-Mg,Fe-Ca,Fe-Al)性能发现[56],载体特性对Fe组分的化学形态(Fe2O3,Fe3O4和Ca2Fe2O5)、分散特性、孔结构、催化活性和抗积碳性能起着非常重要的作用。其中,Fe-Al催化剂作用下达到最高的气化效率61.4%(质量分数),气体产率为598 mL/g,H2产率为217 mL/g,H2与CO物质的量比为2.5。这主要归因于较小活性颗粒粒径,较高比表面积以及Al的引入提供了酸位点,可以为热解挥发分的裂解、重整和水汽变换反应提供更多活性位点。这些研究表明,基于LDHs构筑高活性、高稳定性催化剂在生物质催化热解过程中具有出显著优势。
3 应用前景展望
1) 利用生物质热化学转化获得高品质合成气的关键是焦油的脱除和目标产物H2和CO的选择性。热解过程中生成的大量焦油类化合物不仅浪费能量,而且严重制约合成气的转化应用。为了提高合成气产率和品质,选择合适的催化剂至关重要。然而,催化剂在使用过程中通常存在烧结团聚和表面积碳行为,导致催化活性迅速降低甚至失活。当前学者们主要从催化剂的活性组分调变、载体组成结构修饰和酸碱性优化3方面进行调控,缓解催化剂易烧结、易积碳的问题。添加助剂往往需要以损失活性组分负载量为代价,而增加活性组分负载量又会导致高温团聚等问题。另外,引入多孔、酸性载体虽然能够提高活性组分的分散度和暴露数目,但热解过程中产生的大分子产物容易在活性位点发生缩合反应生成积碳,造成活性位点覆盖和孔道堵塞,增加反应物质扩散阻力,同时阻碍反应物和活性位点的进一步接触,降低催化剂催化活性。因此,设计合成活性位点小尺寸、高负载、高分散且具有一定碱性的多功能催化剂对生物质热化学制高品质合成气是非常有意义的。
2) LDHs作为一种二维层状双金属氢氧化物,具有主体层板金属组成及比例可调、金属离子呈原子水平高度分散、富含羟基等特性,以其作为前驱体经结构拓扑转变可制备得到尺寸小、负载量高、分散性好、热稳定强的碱性金属及金属氧化合物催化剂。LDHs层板组成的可调变有利于根据生物质热转化过程中发生的焦油裂解、水蒸气重整和水煤气变换等多个反应需要设计合成不同活性组分的二元、三元甚至多元金属复合催化剂;焙烧产物的小尺寸、高度分散特性导致催化剂中活性位点保持较高的反应活性且更容易与热解挥发分反应物接触,促进其向小分子气体的高效转化;而载体酸碱特性的调控则可通过调变层板金属种类及比例来实现,进而优化挥发分中含氧有机大分子在活性位点的吸附、脱氧转化路径;LDHs层间阴离子在焙烧过程中发生分解,在气体脱除过程中会在催化剂表面形成丰富的孔道结构,进而促进生物质热转化过程中大分子产物的扩散迁移,抑制积碳的沉积。此外,LDHs也可作为载体,将其他活性金属组分作为外源活性位进行负载,进一步提高催化活性以及稳定性,对LDHs材料的结构性能再次优化。
参考文献:
[1] OUMER A N, HASAN M M, BAHETA A T, et al. Bio-based liquid fuels as a source of renewable energy: a review[J]. Renewable and Sustainable Energy Reviews, 2018, 88: 82-98.
[2] CHEN Weidong, GENG Wenxin. Fossil energy saving and CO2 emissions reduction performance, and dynamic change in performance considering renewable energy input[J]. Energy, 2017, 120: 283-292.
[3] GUAN Guoqing, KAEWPANHA M, HAO Xiaogang, et al. Catalytic steam reforming of biomass tar: Prospects and challenges[J]. Renewable and Sustainable Energy Reviews, 2016, 58: 450-461.
[4] NASIR UDDIN M, DAUD W M A W, ABBAS H F. Potential hydrogen and non-condensable gases production from biomass pyrolysis: Insights into the process variables[J]. Renewable and Sustainable Energy Reviews, 2013, 27: 204-224.
[5] PANDEY B, PRAJAPATI Y K, SHETH P N. Recent progress in thermochemical techniques to produce hydrogen gas from biomass: a state of the art review[J]. International Journal of Hydrogen Energy, 2019, 44(47): 25384-25415.
[6] ZHANG Shuping, ZHU Shuguang, ZHANG Houlei, et al. High quality H2-rich syngas production from pyrolysis-gasification of biomass and plastic wastes by Ni-Fe@Nanofibers/Porous carbon catalyst[J]. International Journal of Hydrogen Energy, 2019, 44(48): 26193-26203.
[7] JIN Fangzhu, SUN Hongman, WU Chunfei, et al. Effect of calcium addition on Mg-AlOx supported Ni catalysts for hydrogen production from pyrolysis-gasification of biomass[J]. Catalysis Today, 2018, 309: 2-10.
[8] KUMAGAI S, ALVAREZ J, BLANCO P H, et al. Novel Ni-Mg-Al-Ca catalyst for enhanced hydrogen production for the pyrolysis-gasification of a biomass/plastic mixture[J]. Journal of Analytical and Applied Pyrolysis, 2015, 113: 15-21.
[9] SINGH H, YADAV R, FAROOQUI S A, et al. Nanoporous nickel oxide catalyst with uniform Ni dispersion for enhanced hydrogen production from organic waste[J]. International Journal of Hydrogen Energy, 2019, 44(36): 19573-19584.
[10] SHI Xunwang, ZHANG Kaidi, CHENG Qunpeng, et al. Promoting hydrogen-rich syngas production through catalytic cracking of rape straw using Ni-Fe/PAC-γAl2O3 catalyst[J]. Renewable Energy, 2019, 140: 32-38.
[11] CHEN Fangyuan, WU Chunfei, DONG Lisha, et al. Characteristics and catalytic properties of Ni/CaAlOx catalyst for hydrogen-enriched syngas production from pyrolysis-steam reforming of biomass sawdust[J]. Applied Catalysis B: Environmental, 2016, 183: 168-175.
[12] YAO Dingding, YANG Haiping, CHEN Hanping, et al. Investigation of nickel-impregnated zeolite catalysts for hydrogen/syngas production from the catalytic reforming of waste polyethylene[J]. Applied Catalysis B: Environmental, 2018, 227: 477-487.
[13] YE Mengjing, TAO Yongwen, JIN Fangzhu, et al. Enhancing hydrogen production from the pyrolysis-gasification of biomass by size-confined Ni catalysts on acidic MCM-41 supports[J]. Catalysis Today, 2018, 307: 154-161.
[14] DONG Lisha, WU Chunfei, LING Huajuan, et al. Promoting hydrogen production and minimizing catalyst deactivation from the pyrolysis-catalytic steam reforming of biomass on nanosized NiZnAlOx catalysts[J]. Fuel, 2017, 188: 610-620.
[15] LI Qingyuan, JI Shengfu, HU Jinyong, et al. Catalytic steam reforming of rice straw biomass to hydrogen-rich syngas over Ni-based catalysts[J]. Chinese Journal of Catalysis, 2013, 34(7): 1462-1468.
[16] 倪哲明, 潘国祥, 王力耕, 等. 二元类水滑石层板组成、结构与性能的理论研究[J]. 无机化学学报, 2006, 22(1): 91-95.
NI Zheming, PAN Guoxiang, WANG Ligeng, et al. Theoretical studies on layer composition, structure and properties of hydrotalcite[J]. Chinese Journal of Inorganic Chemistry, 2006, 22(1): 91-95.
[17] GOH K H, LIM T T, DONG Zhili. Application of layered double hydroxides for removal of oxyanions: a review[J]. Water Research, 2008, 42(6/7): 1343-1368.
[18] KELKAR C P, SCHUTZ A A. Ni-Mg and Co-containing hydrotalcite-like materials with a sheet-like morphology: synthesis and characterization[J]. Microporous Materials, 1997, 10(4/5/6): 163-172.
[19] PALMER S J, FROST R L, NGUYEN T. Hydrotalcites and their role in coordination of anions in Bayer liquors: Anion binding in layered double hydroxides[J]. Coordination Chemistry Reviews, 2009, 253(1/2): 250-267.
[20] TSUJI H, FUJII S. Phosphate recovery by generating hydroxyapatite via reaction of calcium eluted from layered double hydroxides[J]. Applied Clay Science, 2014, 99: 261-265.
[21] ZHANG Ruihong, AI Yuejie, LU Zhanhui. Application of multifunctional layered double hydroxides for removing environmental pollutants: recent experimental and theoretical progress[J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 103908.
[22] CELAYA-SANFIZ A, MORALES-VEGA N, DE MARCO M, et al. Self-condensation of acetone over Mg-Al layered double hydroxide supported on multi-walled carbon nanotube catalysts[J]. Journal of Molecular Catalysis A: Chemical, 2015, 398: 50-57.
[23] MONDAL S, DASGUPTA S, MAJI Kanchan. MgAl- layered double hydroxide nanoparticles for controlled release of salicylate[J]. Materials Science and Engineering: C, 2016, 68: 557-564.
[24] ZHANG Zhiqing, ZENG Hongyan, LIU Xiaojun, et al. Modification of MgAl hydrotalcite by ammonium sulfate for enhancement of lead adsorption[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 60: 361-368.
[25] RAHMAN M T, KAMEDA T, KUMAGAI S, et al. A novel method to delaminate nitrate-intercalated MgAl layered double hydroxides in water and application in heavy metals removal from waste water[J]. Chemosphere, 2018, 203: 281-290.
[26] GRANADOS-REYES J, SALAGRE P, CESTEROS Y. CaAl-layered double hydroxides as active catalysts for the transesterification of glycerol to glycerol carbonate[J]. Applied Clay Science, 2016, 132/133: 216-222.
[27] LIU Jianqiang, SONG Jianye, XIAO Hongdi, et al. Synthesis and thermal properties of ZnAl layered double hydroxide by urea hydrolysis[J]. Powder Technology, 2014, 253: 41-45.
[28] LU Yi, JIANG Bin, FANG Liang, et al. High performance NiFe layered double hydroxide for methyl orange dye and Cr(VI) adsorption[J]. Chemosphere, 2016, 152: 415-422.
[29] ELMOUBARKI R, MAHJOUBI F Z, ELHALIL A, et al. Ni/Fe and Mg/Fe layered double hydroxides and their calcined derivatives: preparation, characterization and application on textile dyes removal[J]. Journal of Materials Research and Technology, 2017, 6(3): 271-283.
[30] WU Hongyu, JIAO Qingze, ZHAO Yun, et al. Synthesis of Zn/Co/Fe-layered double hydroxide nanowires with controllable morphology in a water-in-oil microemulsion[J]. Materials Characterization, 2010, 61(2): 227-232.
[31] TAEI M, HAVAKESHIAN E, HASANPOUR F, et al. Mg-Ca-Fe layered double hydroxide-gold nanoparticles as an efficient electrocatalyst for ethanol oxidation[J]. Journal of the Taiwan Institute of Chemical Engineers, 2016, 67: 184-190.
[32] WANG Duan, WU Jingli, BAI Daxun, et al. Mesoporous spinel ferrite composite derived from a ternary MgZnFe-layered double hydroxide precursor for lithium storage[J]. Journal of Alloys and Compounds, 2017, 726: 306-314.
[33] XIA Shengjie, ZHANG Lianyang, ZHOU Xiaobo, et al. The photocatalytic property for water splitting and the structural stability of CuMgM layered double hydroxides (M=Al, Cr, Fe, Ce)[J]. Applied Clay Science, 2015, 114: 577-585.
[34] XU Zhi ping, ZHANG Jia, ADEBAJO M O, et al. Catalytic applications of layered double hydroxides and derivatives[J]. Applied Clay Science, 2011, 53(2): 139-150.
[35] 余俊, 杨宇森, 卫敏. 水滑石基负载型催化剂的制备及其在催化反应中的应用[J]. 化学学报, 2019, 77(11): 1129-1139.
YU Jun, YANG Yusen, WEI Min. Preparation and catalytic performance of supported catalysts derived from layered double hydroxides[J]. Acta Chimica Sinica, 2019, 77(11): 1129-1139.
[36] ZHAO Mengqiang, ZHANG Qiang, ZHANG Wei, et al. Embedded high density metal nanoparticles with extraordinary thermal stability derived from Guest-Host mediated layered double hydroxides[J]. Journal of the American Chemical Society, 2010, 132(42): 14739-14741.
[37] YAN Qinghua, CHEN Sining, ZHANG Cheng, et al. Synthesis of Cu0.5Mg1.5Mn0.5Al0.5Ox mixed oxide from layered double hydroxide precursor as highly efficient catalysts for low-temperature selective catalytic reduction of NOx with NH3[J]. Journal of Colloid and Interface Science, 2018, 526: 63-74.
[38] LIU Yanan, ZHAO Jiaying, FENG Junting, et al. Layered double hydroxide-derived Ni-Cu nanoalloy catalysts for semi-hydrogenation of alkynes: Improvement of selectivity and anti-coking ability via alloying of Ni and Cu[J]. Journal of Catalysis, 2018, 359: 251-260.
[39] MOSTAJERAN M, PREVOT V, MAL S S, et al. Base-metal catalysts based on porous layered double hydroxides for alkaline-free sodium borohydride hydrolysis[J]. International Journal of Hydrogen Energy, 2017, 42(31): 20092-20102.
[40] RADHA S, NAVROTSKY A. Energetics of CO2 adsorption on Mg-Al layered double hydroxides and related mixed metal oxides[J]. The Journal of Physical Chemistry C, 2014, 118(51): 29836-29844.
[41] BING Weihan, ZHENG Lei, HE Shan, et al. Insights on active sites of CaAl-hydrotalcite as a high-performance solid base catalyst toward aldol condensation[J]. ACS Catalysis, 2018, 8(1): 656-664.
[42] GUO Xinpeng, PENG Zhijian, HU Mingxiang, et al. Highly active Ni-based catalyst derived from double hydroxides precursor for low temperature CO2 methanation[J]. Industrial & Engineering Chemistry Research, 2018, 57(28): 9102-9111.
[43] 安哲, 何静, 段雪. 基于层状前驱体制备活性位高分散催化材料[J]. 催化学报, 2013, 34(1): 225-234.
AN Zhe, HE Jing, DUAN Xue. Catalysts with catalytic sites highly dispersed from layered double hydroxide as precursors[J]. Chinese Journal of Catalysis, 2013, 34(1): 225-234.
[44] LIAO Yunhui, LI Feng, DAI Xin, et al. Solid base catalysts derived from Ca-M-Al (M=Mg, La, Ce, Y) layered double hydroxides for dimethyl carbonate synthesis by transesterification of methanol with propylene carbonate[J]. Chinese Journal of Catalysis, 2017, 38(11): 1860-1869.
[45] GANDARA-LOE J, JACOBO-AZUARA A, SILVESTRE-ALBERO J, et al. Layered double hydroxides as base catalysts for the synthesis of dimethyl carbonate[J]. Catalysis Today, 2017, 296: 254-261.
[46] LIN Weiwei, CHENG Haiyang, LI Xiaoru, et al. Layered double hydroxide-like Mg3Al1-xFex materials as supports for Ir catalysts: Promotional effects of Fe doping in selective hydrogenation of cinnamaldehyde[J]. Chinese Journal of Catalysis, 2018, 39(5): 988-996.
[47] TIAN Zhengbin, LI Qingyang, HOU Juying, et al. Platinum nanocrystals supported on CoAl mixed metal oxide nanosheets derived from layered double hydroxides as catalysts for selective hydrogenation of cinnamaldehyde[J]. Journal of Catalysis, 2015, 331: 193-202.
[48] JIN Qiu, HE Yufei, MIAO Manyu, et al. Highly selective and stable PdNi catalyst derived from layered double hydroxides for partial hydrogenation of acetylene[J]. Applied Catalysis A: General, 2015, 500: 3-11.
[49] CAO Lei, MA Yinghua, SONG Ailing, et al. Stable composite of flower-like NiFe-layered double hydroxide nucleated on graphene oxide as an effective catalyst for oxygen reduction reaction[J]. International Journal of Hydrogen Energy, 2019, 44(12): 5912-5920.
[50] LIANG Zhong, ZHOU Peng, WANG Zeyan, et al. Electrodeposition of NiFe layered double hydroxide on Ni3S2 nanosheets for efficient electrocatalytic water oxidation[J]. International Journal of Hydrogen Energy, 2020, 45(15): 8659-8666.
[51] NAVARRO R M, GUIL-LOPEZ R, FIERRO J L G, et al. Catalytic fast pyrolysis of biomass over Mg-Al mixed oxides derived from hydrotalcite-like precursors: Influence of Mg/Al ratio[J]. Journal of Analytical and Applied Pyrolysis, 2018, 134: 362-370.
[52] WANG Lei, LI Dalin, WATANABE H, et al. Catalytic performance and characterization of Co/Mg/Al catalysts prepared from hydrotalcite-like precursors for the steam gasification of biomass[J]. Applied Catalysis B: Environmental, 2014, 150/151: 82-92.
[53] WANG Lei, CHEN Jinhai, WATANABE H, et al. Catalytic performance and characterization of Co-Fe bcc alloy nanoparticles prepared from hydrotalcite-like precursors in the steam gasification of biomass-derived tar[J]. Applied Catalysis B: Environmental, 2014, 160/161: 701-715.
[54] YANG Shuangxia, ZHANG Xiaodong, CHEN Lei, et al. Characteristics and catalytic properties of Bi-functional Fe/CaO catalyst for syngas production from pyrolysis of biomass[J]. Journal of Biobased Materials and Bioenergy, 2016, 10(6): 415-422.
[55] ZHANG Xiaodong, YANG Shuangxia, XIE Xinping, et al. Stoichiometric synthesis of Fe/CaxO catalysts from tailored layered double hydroxide precursors for syngas production and tar removal in biomass gasification[J]. Journal of Analytical and Applied Pyrolysis, 2016, 120: 371-378.
[56] YANG Shuangxia, ZHANG Xiaodong, CHEN Lei, et al. Pyrolysis of sawdust with various Fe-based catalysts: Influence of support characteristics on hydrogen-rich gas production[J]. Journal of Analytical and Applied Pyrolysis, 2019, 137: 29-36.
[57] XU Changchun, CHEN Shiyi, SOOMRO A, et al. Hydrogen rich syngas production from biomass gasification using synthesized Fe/CaO active catalysts[J]. Journal of the Energy Institute, 2018, 91(6): 805-816.
[58] HUANG Xiangneng, WU Jiawei, WANG Mingfeng, et al. Syngas production by chemical looping gasification of rice husk using Fe-based oxygen carrier[J]. Journal of the Energy Institute, 2020, 93(4): 1261-1270.
(编辑 秦明阳)
收稿日期: 2020 -11 -16; 修回日期: 2021 -01 -20
基金项目(Foundation item):山东省自然科学基金资助项目(ZR2018MEE029);山东省高等学校青年创新团队项目(2019KJD002);济南市科技计划项目(201913013);山东省生物工程技术创新中心重大创新项目(2019JSWGCCXZX002);国家自然科学基金资助项目(51876108) (Project(ZR2018MEE029) supported by the Natural Science Foundation of Shandong Province; Project(2019KJD002) supported by the Shandong Province University Youth Innovation Team Program; Project(201913013) supported by the Jinan Science and Technology Program; Project(2019JSWGCCXZX002) supported by the Major innovation Program of Shandong Bioengineering Technology Innovation Center; Project(51876108) supported by the National Natural Science Foundation of China)
通信作者:华栋梁,博士,研究员,从事生物质能源与资源利用;E-mail: huadl@sderi.cn
DOI: 10.11817/j.issn.1672-7207.2021.06.034
引用格式:赵文祥, 杨双霞, 陈雷, 等. 层状双金属氢氧化物催化生物质热解制高品质合成气应用前景展望[J]. 中南大学学报(自然科学版), 2021, 52(6): 2040-2051.
Citation:ZHAO Wenxiang, YANG Shuangxia, CHEN Lei, et al. Prospect of layered double hydroxide for high quality syngas production from catalytic pyrolysis of biomass[J]. Journal of Central South University(Science and Technology), 2021, 52(6): 2040-2051.
摘要:合成气是制备生物质基液体燃料和高附加值化学品的重要原料,热解是实现生物质在低温下向高品质合成气转化的有效途径之一。在热解过程中,催化剂能够促进生物质热解挥发分中焦油大分子的裂解、重整转化,降低CO2和CH4等气体组分体积分数,同时通过水汽变换反应调整气体组分,最终实现合成气的净化和调变,而高活性、热稳定和抗积碳催化剂的设计研发始终是生物质热转化过程的研究热点和难点。针对生物质热转化过程,本文综述了催化剂活性组分调变、载体组成结构修饰和物化特性优化3个方面最新研究进展。针对催化剂在反应过程中易高温烧结、积碳失活问题,结合层状双金属氢氧化物(LDHs,水滑石)材料独特的层板元素比例可调、金属阳离子呈原子水平分散、物化结构特性以及其作为催化剂前体/载体在多相催化领域的优异性能,展望了LDHs材料在生物质催化热解制高品质合成气的应用前景。以LDHs作为催化剂前体,经拓扑结构转变制备的金属/金属氧化物复合材料(M/MMO)可实现活性组分的高度分散和酸碱性调控,进而有效解决热转化过程中催化剂高温烧结问题,并抑制积碳形成,促进生物质向高品质合成气的高效、稳定转化。


