Trans. Nonferrous Met. Soc. China 26(2016) 1055-1062
Thermodynamic assessment of Ni-Yb binary system
Dai-man ZHU1, Chang-rong LI1, Cui-ping GUO1, Zhen-min DU1, Jun-qin LI2
1. School of Materials Science and Engineering, University of Science and Technology Beijing, Beijing 100083, China;
2. Shenzhen Key Laboratory of Special Functional Materials, Shenzhen University, Shenzhen 518060, China
Received 16 April 2015; accepted 26 August 2015
Abstract:
On the basis of the experimental data of phase equilibria and thermochemical properties available from literatures, a critical assessment for the Ni-Yb binary system was carried out using the CALPHAD (calculation of phase diagrams) method. The liquid phase is modeled as the associate model with the constituent species Ni, Yb and YbNi3, owing to the sharp change of the enthalpy of mixing of liquid phase at the composition of around 25% Yb (mole fraction). The terminal solid solutions FCC_A1 (Ni/Yb) and BCC_A2 (Yb) are described by the substitutional solution model with the Redlich-Kister polynomial. The intermetallic compounds, Yb2Ni17, YbNi5, YbNi3, YbNi2, α-YbNi and β-YbNi, are treated as strict stoichiometric compounds, since there are no noticeable homogeneity ranges reported for these compounds. A set of self-consistent thermodynamic parameters for the Ni-Yb binary system are obtained. According to the presently assessed results, the thermochemical properties and the phase boundary data can be well reproduced.
Key words:
Ni-Yb system; thermodynamic assessment; CALPHAD technique;
1 Introduction
During the last few decades, the investigation of intermetallic compounds between rare earth (RE) and transition metals has attracted a great deal of attention. In the Ni-RE series, the Ni-Yb system may be of special interest for its extensive applications as thermoelectric, amorphous and catalytic materials. The Ni electrode is a good electrode candidate for the filled skutterudite Yb0.3Co4Sb12 thermoelectric devices working in air at high temperature [1]. The aluminum-nickel-ytterbium (Al-Ni-Yb) alloys, with the Al-rich precipitates and the Ni and Ni-Yb clusters coexisted, have good glass- forming abilities of metallic glasses with excellent mechanical properties [2,3]. The Ni-Yb/SiO2 and Ni/Yb2O3 catalysts deliver high conversions for the reaction of gas phase phenol hydrogenation and the oxidation of CH4, respectively [4,5]. Therefore, to better understand the thermochemical properties and the phase equilibrium relations concerning the Ni-RE binary systems will play an important role in the development of Ni-based materials with RE elements, and the Ni-Yb binary system is one of such systems.
According to the literature review, there have been one evaluation and one computation for the Ni-Yb binary system so far [6,7]. The former [6] summarized the experimental work conducted by PALENZONA and CIRAFICI [8] using X-ray diffraction (XRD), differential thermal analysis (DTA) and metallography measurement. The latter [7] reported the calculated Ni-Yb phase diagram besides the measured data of the mixing enthalpy of the liquid phase, but neither the assessed method nor the optimized parameters were reported. More importantly, the phase diagram presented by Ref. [7] is much different from the one determined experimentally by PALENZONA and CIRAFICI [8].
The present work is to assess the Ni-Yb binary system thermodynamically by the calculation of phase diagrams (CALPHAD) method, mainly considering the phase equilibrium data in Ref. [8], the phase diagram evaluation in Ref. [6], the enthalpies of formation of the compounds in Ref. [9] and the mixing enthalpy of the liquid phase in Ref. [7]. The self-consistent thermo- dynamic parameters are optimized for calculating the phase equilibria and the thermochemical properties of the Ni-Yb binary system.
2 Evaluation of available information
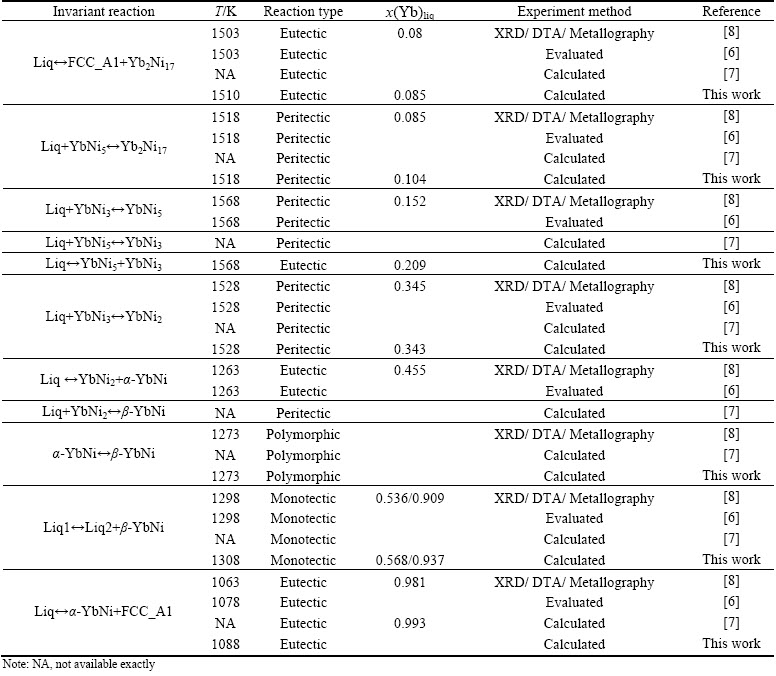
The experimental information of Ni-Yb system is limited. Using XRD, BUSCHOW [10] firstly studied the five intermetallic compounds, YbNi, YbNi2, YbNi3, YbNi5 and Yb2Ni17, existed in the system. Later on, PALENZONA and CIRAFICI [8] measured the phase diagram in the whole composition range by XRD, differential thermal analysis (DTA) and metallography. In Ref. [8], the β-YbNi and YbNi3 compounds melt congruently at 1308 and 1583 K, respectively, while β-YbNi shows a polymorphic transformation of α-YbNi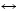 β-YbNi at the solid state and 1273 K. The compounds YbNi2, YbNi5 and Yb2Ni17 are formed by the peritectic reactions L+YbNi3
β-YbNi at the solid state and 1273 K. The compounds YbNi2, YbNi5 and Yb2Ni17 are formed by the peritectic reactions L+YbNi3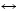 YbNi2 at 1528 K, L+YbNi3
YbNi2 at 1528 K, L+YbNi3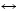 YbNi5 at 1568 K and L+YbNi5
YbNi5 at 1568 K and L+YbNi5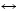 Yb2Ni17 at 1518 K, respectively. Three eutectic transformations were defined, Liq
Yb2Ni17 at 1518 K, respectively. Three eutectic transformations were defined, Liq FCC_A1+Yb2Ni17 at 1503 K and ~7.5% Yb (mole fraction), Liq
FCC_A1+Yb2Ni17 at 1503 K and ~7.5% Yb (mole fraction), Liq YbNi2+ α-YbNi at 1263 K and ~46.0% Yb, and Liq
YbNi2+ α-YbNi at 1263 K and ~46.0% Yb, and Liq α-YbNi+ FCC_A1 at 1063 K and ~98.0% Yb. According to Ref. [8], from about 55% to 90% Yb, Yb and YbNi give rise to an miscibility gap at liquid state, which extends well above 1298 K. The Ni-Yb alloys in this composition range are always two-layered after rapidly cooled by water quenching from 1773 K to room temperature. The different metallographic images of the two layers from the same sample can be considered to be the microstructures formed from the two immiscible Liquids 1 and 2 with different mass densities. Based on the metallographic images of the sample with 75% Yb quenched from 1773 K and the densities of Yb (6.97 g/cm3) and YbNi (10.08 g/cm3) [8], the compositions of the Yb-rich Liquid 1 (the upper part of the solidified alloy) and the YbNi-rich Liquid 2 (the lower part) were approximately calculated, which are 85% and 66% Yb, respectively. The details of the invariant reactions in the Ni-Yb binary system are shown in Table 1.
α-YbNi+ FCC_A1 at 1063 K and ~98.0% Yb. According to Ref. [8], from about 55% to 90% Yb, Yb and YbNi give rise to an miscibility gap at liquid state, which extends well above 1298 K. The Ni-Yb alloys in this composition range are always two-layered after rapidly cooled by water quenching from 1773 K to room temperature. The different metallographic images of the two layers from the same sample can be considered to be the microstructures formed from the two immiscible Liquids 1 and 2 with different mass densities. Based on the metallographic images of the sample with 75% Yb quenched from 1773 K and the densities of Yb (6.97 g/cm3) and YbNi (10.08 g/cm3) [8], the compositions of the Yb-rich Liquid 1 (the upper part of the solidified alloy) and the YbNi-rich Liquid 2 (the lower part) were approximately calculated, which are 85% and 66% Yb, respectively. The details of the invariant reactions in the Ni-Yb binary system are shown in Table 1.
Table 1 Invariant reactions in Ni-Yb system
BRUTTI et al [9] reported the enthalpies of formation at 298 K of the solid compounds Yb2Ni17, YbNi5, YbNi3, YbNi2 and α-YbNi by tensimetric measurements (Knudsen effusion (KE)-mass spectrometry and Knudsen effusion-weight loss) and calorimetric measurements (direct reaction calorimetry). Using direct synthesis calorimetry (DSC), BORZONE et al [11] tested the enthalpy of formation of the YbNi2 phase, which is larger than that obtained by BRUTTI et al [9]. NIESSEN et al [12] and BAYANOV [13] calculated the values of the enthalpies of formation of all of the compounds of the system. Those data available from literatures are collected in Table 2.
Table 2 Enthalpies of formation of intermetallic compounds in Ni-Yb system
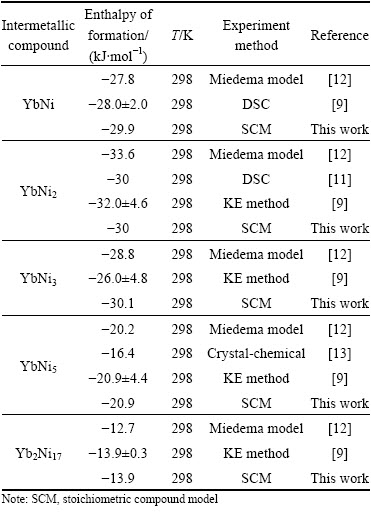
Recently, BEREZUTSKII et al [7] investigated the mixing enthalpy of melts of the Ni-Yb binary system at 1300-1750 K using the isoperibolic calorimetry. Then, they constructed the models of the liquid and solid alloys and calculated the phase diagram which is much different from the one determined experimentally by PALENZONA and CIRAFICI [8]. On the basis of the experimental measurement, Ref. [7] proposed some suspicious points about the doubtful questions to the shape of liquidus and the melting points of compounds without further experimental confirmation.
In the present assessment of Ni-Yb binary system, the phase equilibrium data in Ref. [8], the phase diagram evaluation in Ref. [6], the enthalpies of formation of the compounds in Ref. [9] and the mixing enthalpy of the liquid phase in Ref. [7] are mainly under consideration with high weighting factors. The comment in Ref. [7] is also taken into account that the most refractory compound in the Ni-Yb binary system should be YbNi5 most probably since LnNi5 is the most refractory compound in all other Ni-Ln systems [14-16].
3 Thermodynamic models
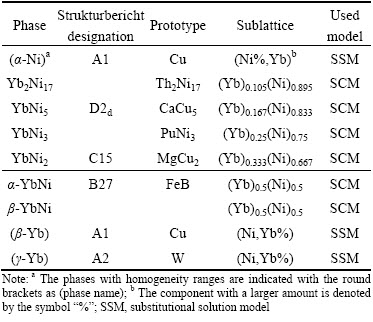
The information about the stable solid phases of Ni-Yb binary system [8], including the prototypes, the sublattices and the models used in the present assessment, is listed in Table 3.
Table 3 Stable solid phases and used models in Ni-Yb binary system
3.1 Pure elements
For the function of Gibbs free energy for a pure element, the reference state is the enthalpy of the element in its most stable form at the standard state pressure and 298.15 K. The Gibbs free energy of the pure element i in its stable or metastable state of the phase φ is described by an equation of the following form:
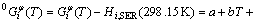
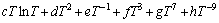 (1)
(1)
where i represents the pure element Ni or Yb; Hi,SER (298.15 K) is the molar enthalpy of the element i at 298.15 K in its standard element reference (SER) state, FCC_A1 for both Ni and Yb; and a to h are the coefficients. The molar Gibbs free energy of the element i, in its SER state, is denoted by Gi,SER as follows:
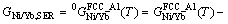
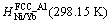 (2)
(2)
The molar Gibbs free energy functions of pure elements are taken from the SGTE (Scientific Group Thermodata Europe) compiled by DINSDALE [17].
3.2 Solution phases
3.2.1 Terminal solid solution phases
The molar Gibbs free energies of FCC_A1 and BCC_A2 phases in the Ni-Yb binary system are described by the substitutional solution model (SSM) as follows:

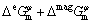 (3)
(3)
where xi is the mole fraction of the pure element i,  is the molar Gibbs free energy of the pure element i in the state of φ phase, which is taken from the SGTE database [16], R is the mole gas constant, T is the thermodynamic temperature,
is the molar Gibbs free energy of the pure element i in the state of φ phase, which is taken from the SGTE database [16], R is the mole gas constant, T is the thermodynamic temperature, 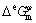 is the excess molar Gibbs free energy, and
is the excess molar Gibbs free energy, and 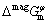 is the magnetic contribution to the molar Gibbs free energy which equals zero if there is no magnetic contribution, such as Yb.
is the magnetic contribution to the molar Gibbs free energy which equals zero if there is no magnetic contribution, such as Yb.
The excess Gibbs free energy is written as a Redlich-Kister polynomial [18]:
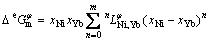 (4)
(4)
where 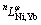 is the nth interaction parameter between the components Ni and Yb, which is the function of temperature:
is the nth interaction parameter between the components Ni and Yb, which is the function of temperature:

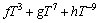 (5)
(5)
In most cases, only the first one or two terms of the above equation are used.
The magnetic contribution is considered for the phase (α-Ni). It is described according to the model proposed by HILLERT and JARL [19]:
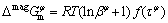 (6)
(6)
where βφ is the Bothr magneton number, f(τφ) is a polynomial with 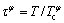 , and
, and 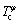 is the Curie temperature of solution for ferromagnetic ordering. βφ and
is the Curie temperature of solution for ferromagnetic ordering. βφ and 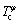 for the phase (α-Ni) are taken from the SGTE database [17].
for the phase (α-Ni) are taken from the SGTE database [17].
3.2.2 Liquid phase
For the Ni-Yb binary system, the diagram of the enthalpy of mixing of liquid phase constructed by BEREZUTSKII et al [7] is characterized by a sharp change around the compound YbNi3. This implies that some types of complex or associate are formed inside the liquid at this critical composition [20]. Therefore, the associate model [21] was proposed for the liquid phase description with the constituent species of Ni, Yb and YbNi3. The molar Gibbs free energy is expressed with the following expression [18]:
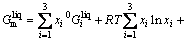
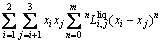 (7)
(7)
where 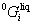 and xi are the molar Gibbs free energy and the mole fraction of the pure species i (i=1,2,3 represent Ni, YbNi3 and Yb, respectively) at the liquid state;
and xi are the molar Gibbs free energy and the mole fraction of the pure species i (i=1,2,3 represent Ni, YbNi3 and Yb, respectively) at the liquid state; 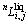 is the nth interaction parameter (n=0,1,2 in the present work) between the species i and j (j=2,3 represent YbNi3 and Yb, respectively at the liquid state), which is a function of temperature as
is the nth interaction parameter (n=0,1,2 in the present work) between the species i and j (j=2,3 represent YbNi3 and Yb, respectively at the liquid state), which is a function of temperature as 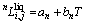 and the coefficients an and bn are the parameters to be optimized.
and the coefficients an and bn are the parameters to be optimized.
3.3 Stoichiometric compounds
The intermetallic compounds YbNi, YbNi2, YbNi3, YbNi5 and Yb2Ni17 in the Ni-Yb binary system are stoichiometric phases. YbNi undergoes a polymorphic transformation between the low and high temperature phases, α-YbNi and β-YbNi. The Gibbs free energy per mole atom of YbpNiq is expressed as follows:
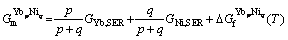 (8)
(8)
where 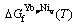 is the Gibbs free energy of formation of the stoichiometric compound YbpNiq from the pure elements in their SER states. Owing to no thermochemical measurements available for these compounds, it is assumed that the Neumann-Kopp rule [22] applies to the heat capacity description, i.e., ΔCp=0. Thus,
is the Gibbs free energy of formation of the stoichiometric compound YbpNiq from the pure elements in their SER states. Owing to no thermochemical measurements available for these compounds, it is assumed that the Neumann-Kopp rule [22] applies to the heat capacity description, i.e., ΔCp=0. Thus, 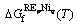 is described as
is described as
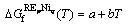 (9)
(9)
where the coefficients a and b are the parameters to be optimized.
4 Results and discussion
With the lattice stabilities of all pure elements from the study of DINSDALE [17] and the PARROT module of the Thermo-Calc software [23,24], the phase relations and the thermochemical properties of the Ni-Yb binary system were carefully assessed based on the experimental data available from literatures.
The parameters were optimized step by step. It works by minimizing an error of sum with each piece of selected information given a certain weight. The weight was given and adjusted based on the original data uncertainty and the personal evaluation. Based on the experimental thermochemical and phase equilibrium data, the parameters related to the solution phases, the liquid phase and the terminal solid solution phases, are optimized firstly. A higher weight is given to the equilibria related to the miscibility gap than others when optimizing the liquid phase, to make the miscibility gap exist at the Yb-rich side. Then, the intermetallic compound phases are optimized according to their enthalpies of formation to match their equilibrium relations.
Table 4 Thermodynamic parameters of Ni-Yb binary system
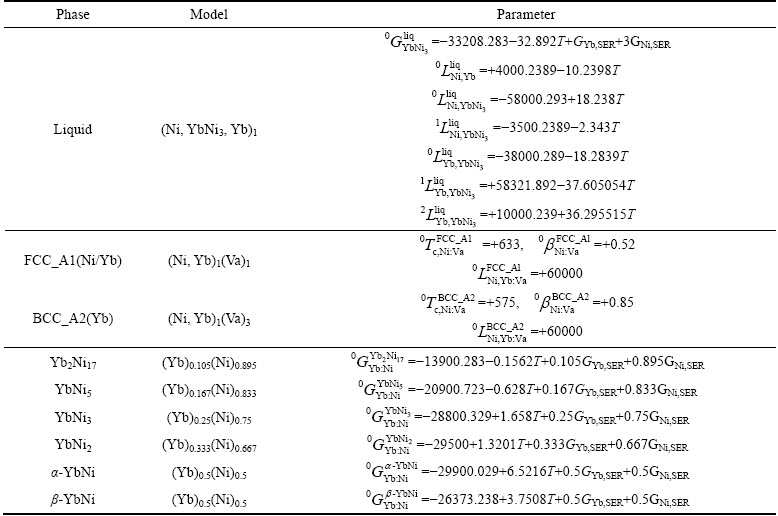
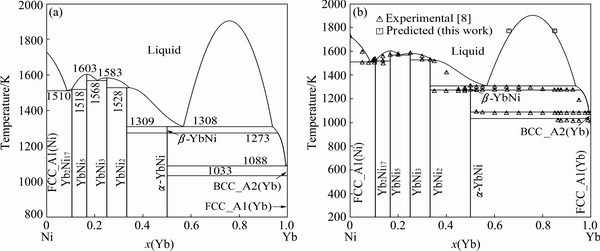
Fig. 1 Ni-Yb binary phase diagram calculated with present description (a) and compared with experimental data (b)
Table 4 lists the optimized thermodynamic parameters for the Ni-Yb binary system. Using these parameters, the calculations can well reproduce most of the thermochemical properties and phase boundary data. For checking the results, the phase diagram of the Ni-Yb binary system was also calculated using Pandat software [25].
The calculated phase diagram is shown in Fig. 1 together with the experimental data obtained by PALENZONA and CIRAFICI [8] and all the invariant temperatures labeled. The reversible polymorphic transition of the compound YbNi, α-YbNi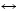 β-YbNi, is at 1273 K. The calculated incongruent melting temperatures of the intermetallic compounds Yb2Ni17, YbNi2 and β-YbNi are 1518, 1528 and 1309 K, respectively, and the calculated congruent melting temperatures of YbNi5 and YbNi3 are 1603 and 1583 K, respectively. The eutectic reaction temperatures for Liq
β-YbNi, is at 1273 K. The calculated incongruent melting temperatures of the intermetallic compounds Yb2Ni17, YbNi2 and β-YbNi are 1518, 1528 and 1309 K, respectively, and the calculated congruent melting temperatures of YbNi5 and YbNi3 are 1603 and 1583 K, respectively. The eutectic reaction temperatures for Liq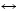 FCC_A1(Ni)+Yb2Ni17, Liq
FCC_A1(Ni)+Yb2Ni17, Liq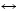 YbNi5+YbNi3 and Liq
YbNi5+YbNi3 and Liq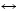 α-YbNi+BCC_A2(Yb) are 1510, 1568, and 1088 K, respectively. A good agreement between the temperatures of the present calculated and the experimentally determined invariant reactions is obtained.
α-YbNi+BCC_A2(Yb) are 1510, 1568, and 1088 K, respectively. A good agreement between the temperatures of the present calculated and the experimentally determined invariant reactions is obtained.
There is a lack of experimental data for the top boundary of the liquid miscibility gap between YbNi and Yb, and the only support is the present prediction at 1773 K based on the metallographic images reported in Ref. [8]. The phase equilibrium measurements about this region need further clarification for a better assessment of the system. The rest of the phase diagram shows very good agreement with the experimental data.
The enlargements of the Ni-Yb phase diagram around compounds YbNi5 and YbNi2 are shown in Figs. 2 and 3, respectively. When calculating Fig. 2, the parameter related to the enthalpy of formation of the compound YbNi5, denoted by a, is first fixed to fit the experimental data in Ref. [9]. Then, the parameter related to the entropy of formation of YbNi5, denoted by b, is given different values to find the appropriate one to accord with the experimentally determined solidification temperatures of the compounds YbNi5 and Yb2Ni17 in Ref. [8], i.e., their related invariant reaction temperatures, 1568 K for the peritectic reaction Liq+YbNi3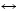 YbNi5 and 1518 K for the peritectic reaction Liq+YbNi5
YbNi5 and 1518 K for the peritectic reaction Liq+YbNi5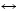 Yb2Ni17. The similar way is used to calculate the data in Fig. 3 to determine approximately the liquidus around the compound YbNi2. Figure 2 shows that the melting temperature (Tm) of YbNi5 is selected as 1603 K, which is 20 K higher than that of the YbNi3. The reasons are the following three points: 1) the calculated formation enthalpy of the compound YbNi5 needs to match the experimental data measured in Ref. [9]; 2) the temperature of the invariant eutectic reaction between the compounds YbNi5 and YbNi3 was determined experimentally to be 1568 K in Ref. [8]; 3) the melting point of YbNi5 is probably higher than that of YbNi3 as Ref. [7] suggested. As shown in Fig. 2, if the two-phase region of Liq+YbNi5 is changed to be broader to accord with the experimental data in Ref. [8], the temperature of the invariant reaction Liq
Yb2Ni17. The similar way is used to calculate the data in Fig. 3 to determine approximately the liquidus around the compound YbNi2. Figure 2 shows that the melting temperature (Tm) of YbNi5 is selected as 1603 K, which is 20 K higher than that of the YbNi3. The reasons are the following three points: 1) the calculated formation enthalpy of the compound YbNi5 needs to match the experimental data measured in Ref. [9]; 2) the temperature of the invariant eutectic reaction between the compounds YbNi5 and YbNi3 was determined experimentally to be 1568 K in Ref. [8]; 3) the melting point of YbNi5 is probably higher than that of YbNi3 as Ref. [7] suggested. As shown in Fig. 2, if the two-phase region of Liq+YbNi5 is changed to be broader to accord with the experimental data in Ref. [8], the temperature of the invariant reaction Liq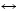 YbNi5+YbNi3 increases much higher than the experimental one. Figure 3 shows the variation of the liquidus line around the compound YbNi2 with the change of its entropy of formation. The thermodynamic parameters of YbNi2 are determined with the consideration of the following three points: 1) the assessed mixing enthalpy of the liquid phase around the compound YbNi2 is consistent with the experimental data determined in Ref. [7]; 2) the assessed temperature of the invariant reaction L+YbNi3
YbNi5+YbNi3 increases much higher than the experimental one. Figure 3 shows the variation of the liquidus line around the compound YbNi2 with the change of its entropy of formation. The thermodynamic parameters of YbNi2 are determined with the consideration of the following three points: 1) the assessed mixing enthalpy of the liquid phase around the compound YbNi2 is consistent with the experimental data determined in Ref. [7]; 2) the assessed temperature of the invariant reaction L+YbNi3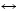 YbNi2 is 1528 K which is the same as the one determined experimentally in Ref. [8]; 3) the assessed formation enthalpies of the compound YbNi2 are in good agreement with the experimental values measured in Ref. [9]. As shown in Fig. 3, if the two-phase region of YbNi2+Liq is changed to be narrower to accord with the experimental data in Ref. [8], the temperature of the peritectic reaction (Tp) Liq+YbNi3
YbNi2 is 1528 K which is the same as the one determined experimentally in Ref. [8]; 3) the assessed formation enthalpies of the compound YbNi2 are in good agreement with the experimental values measured in Ref. [9]. As shown in Fig. 3, if the two-phase region of YbNi2+Liq is changed to be narrower to accord with the experimental data in Ref. [8], the temperature of the peritectic reaction (Tp) Liq+YbNi3 YbNi2 decreases much lower than the experimental one.
YbNi2 decreases much lower than the experimental one.
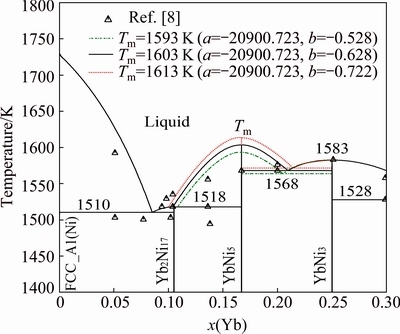
Fig. 2 Calculated enlargement of Ni-Yb phase diagram around compound YbNi5
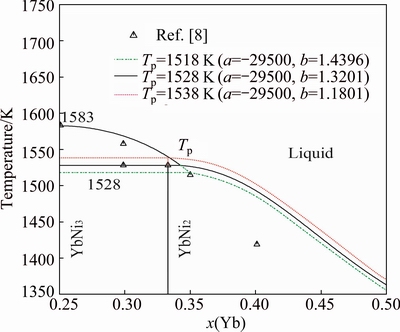
Fig. 3 Calculated enlargement of Ni-Yb phase diagram around compound YbNi2
Figure 4 gives the calculated mixing enthalpy of the liquid phase at 1500 K, which shows good agreement with the experimental data measured at 1750, 1600, 1480 and 1300 K [7], and the asymmetry enthalpy line around the stoichiometric compound YbNi3 implies the reasonable selection of the constituent species of the liquid phase as Ni, Yb and YbNi3. The calculated fractions of the species Ni, YbNi3 and Yb in the liquid phase at 1073, 1773 and 2473 K are shown in Fig. 5. The fraction of YbNi3 increases with the decrease of temperature, and passes through a maximum of approximately 40% at compositions of around 30% Yb.
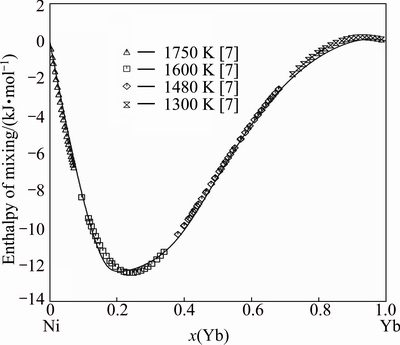
Fig. 4 Enthalpy of mixing of liquid Ni-Yb solution at 1500 K with reference states, Ni (liquid) and Yb (liquid)
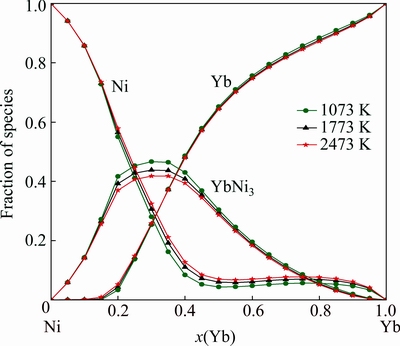
Fig. 5 Calculated fractions of species in liquid phase at 1073, 1773 and 2447 K as function of Yb content
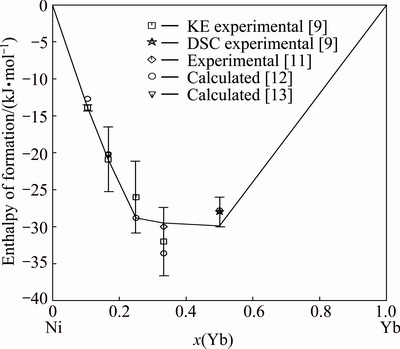
Fig. 6 Enthalpies of formation of intermetallic compounds in Ni-Yb binary system at 298 K with reference states, Ni (FCC_A1) and Yb (FCC_A1)
Figure 6 shows the calculated enthalpies of formation of all intermetallic compounds at 298 K, which are compared with the experimental data [9,11] and the predicted data [12,13]. The calculated values agree very well with the experimental results.
5 Conclusions
1) In order to describe reasonably the sharp change of the enthalpy of mixing of the liquid phase around the stoichiometric compound YbNi3 in the Ni-Yb binary system, the associate model is used and the results are in good agreement with experimental data achieved.
2) There is no direct experimental data available for the top boundary of the liquid miscibility gap between YbNi and Yb. The metallographic images of the upper and lower parts of the same sample obtained by rapid cooling are considered to be the microstructures formed from the two immiscible Liquids 1 and 2 with different mass densities, from which the boundary of the liquid miscibility gap at the experimental annealing temperature of 1773 K is determined.
3) A set of self-consistent thermodynamic parameters for the Ni-Yb binary system are obtained. The comprehensive comparisons show that the calculated phase diagrams and the thermochemical properties agree reasonably with the available experimental data.
Acknowledgements
The authors thanks the Royal Institute of Technology Sweden and CompuTherm LLC for supplying Thermo-Calc software and Pandat Software packages, respectively.
References
[1] FAN X C, GU M, SHI X, CHEN L D, BAI S Q, NUNNA R. Fabrication and reliability evaluation of Yb0.3Co4Sb12/Mo-Ti/Mo- Cu/Ni thermoelectric joints [J]. Ceram Int, 2015, 41: 7590-7595.
[2] JIA R, BIAN X F,  X Q , SONG K K, LI X L. The relationship between viscosity and glass forming ability of Al-(Ni)-Yb alloy systems [J]. Sci China Phys Mech, 2010, 53(3): 390-393.
X Q , SONG K K, LI X L. The relationship between viscosity and glass forming ability of Al-(Ni)-Yb alloy systems [J]. Sci China Phys Mech, 2010, 53(3): 390-393.
[3] ISHEIM D, SEIDMAN D N, PEREPEZKO J H, OLSON G B. Nanometer-scale solute clustering in aluminum/nickel/ytterbium metallic glasses [J]. Mater Sci Eng A, 2003, 353(1): 99-104.
[4] SHORE S G, DING E, PARK C, KEANE M A. The application of {(DMF)10Yb2[TM(CN)4]3}∞(TM=Ni, Pd) supported on silica to promote gas phase phenol hydrogenation[J]. J Mol Catal A: Chem, 2004, 212: 291-300.
[5] DISSANAYAKE D, ROSYNEK M P, LUNSFORD J H. Are the equilibrium concentrations of CO and H2 exceeded during the oxidation of CH4 over a Ni/Yb2O3 catalyst? [J]. J Phys Chem, 1993, 97: 3644-3646.
[6] NASH P. The Ni-Yb (nickel-ytterbium) system [J]. Bull Alloy Phase Diagrams, 1989, 10(2): 129-132.
[7] BEREZUTSKII V V, SHEVCHENKO M A, IVANOV M I, SUDAVTSOVA V S. Thermodynamic properties of liquid alloys Ni-Eu and Ni-Yb [J]. Russ J Phys Chem A, 2014, 88(9): 1463-1471.
[8] PALENZONA A, CIRAFICI S. The ytterbium-nickel system [J]. J Less-Comm Met, 1973, 33(3): 361-367.
[9] BRUTTI S, CICCIOLI A, BALDUCCI G, GIGLI G, BORZONE G, RAGGIO R, FERRO R. Thermodynamics of the Ni-Yb system [J]. J Phase Equilibria, 2002, 23(1): 51-56.
[10] BUSCHOW K H J. Note on the structure and occurrence of ytterbium transition metal compounds [J]. J Less-Comm Met, 1972, 26(3): 329-333.
[11] BORZONE G, FERRO R, PARODI N, SACCONE A. Ytterbium bismuthides-ytterbium valency and thermodynamics [J]. Gazzetta Chimica Italiana, 1995, 125(6): 263-270.
[12] NIESSEN A K, de BOER F R, BOOM R, de CHATEL P F, MATTENS W C M, MIEDEMA A R. Model predictions for the enthalpy of formation of transition metal alloys II [J]. Calphad, 1983, 7(1): 51-70.
[13] BAYANOV A P. Calculation of formation enthalpies of rare earth compounds on the basis of crystal chemical characteristics [J]. Izv Akad Nauk SSSR, Neorg Mater, 1973, 9: 959-963.
[14] BUSCHOW K H, van MAL H H. Phase relations and hydrogen absorption in the lanthanum-nickel system [J]. J Less-Comm Met, 1972, 29(2): 203-210.
[15] GSCHNEIDNER K A Jr, VERKADE M E. Selected cerium phase diagrams [R]. Ames, USA: Rare-Earth Information Center, 1974.
[16] PAN Y Y, ZHENG J X, LI M, YANG H. A phase diagram of the alloys of the Gd-Ni binary system [J]. Acta Phys Sin, 1986, 35(5): 677-680.
[17] DINSDALE A T. SGTE data for pure elements [J]. Calphad, 1991, 15(4): 317-425.
[18] REDLICH O, KISTER A T. Thermodynamics of nonelectrolyte solutions—x-y-t relations in a binary system [J]. Ind Eng Chem, 1948, 40(2): 341-345.
[19] HILLERT M, JARL M. A model for alloying in ferromagnetic metals [J]. Calphad, 1978, 2(3): 227-238.
[20] SAUNDERS N, MIODOWNIK A P. CALPHAD (Calculation of phase diagrams): A comprehensive guide [M]. Vol. 1. Oxford, UK: Elsevier Science Ltd, 1998.
[21] GERLING U, POOL M J, PREDEL B. A contribution to the associate model for binary liquid alloys [J]. Z Metallkd, 1983, 74(9): 616-619.
[22] KOPP H. Investigations of the specific heat of solid bodies [J]. Phil Trans R Soc Lond, 1865: 71-202.
[23] JANSSON B. Computer operated methods for equilibrium calculations and evaluation of thermochemical model parameters [D]. Stockholm, Sweden: Royal Institute of Technology, 1984.
[24] SUNDMAN B, JANSSON B, ANDERSSON J O. The thermo-calc databank system [J]. Calphad, 1985, 9(2): 153-190.
[25] CHEN S L, DANIEL S, ZHANG F, CHANG Y A, YAN X Y, XIE F Y, SCHMID-FETZER R, OATES W A. The PANDAT software package and its applications [J]. Calphad, 2002, 26(2): 175-188.
Ni-Yb二元系的热力学评估
朱代漫1,李长荣1,郭翠萍1,杜振民1,李均钦2
1. 北京科技大学 材料科学与工程学院,北京 100083;
2. 深圳大学 深圳特殊功能材料重点实验室,深圳 518060
摘 要:基于文献报道的相平衡和热化学实验数据,利用相图计算(Calphad)方法对Ni-Yb二元系进行热力学评估。考虑到液相混合焓在25% Yb(摩尔分数)附近有急剧变化,液相采用缔合物模型,组份为Ni、YbNi3和Yb;端际固溶体相包括FCC_A1 (Ni)、FCC_A1 (Yb)和BCC_A2 (Yb),均采用亚规则溶体模型,并按照Redlich-Kister多项式进行描述;中间化合物Yb2Ni17、YbNi5、YbNi3、YbNi2、α-YbNi和β-YbNi都没有明显的固溶度实验数据,均按严格计量比处理。优化得到的Ni-Yb二元系热力学参数自洽合理,能够很好地再现该体系的热化学性质和相图数据。
关键词: Ni-Yb二元系;热力学评估;相图计算技术
(Edited by Mu-lan QIN)
Foundation item: Project (51271027) supported by the National Natural Science Foundation of China; Project (T201308) supported by Shenzhen Key Laboratory of Special Functional Materials of Shenzhen University, China
Corresponding author: Chang-rong LI; Tel: +86-10-82377789; Fax: 86-10-62333772; E-mail: crli@mater.ustb.edu.cn
DOI: 10.1016/S1003-6326(16)64202-4
Abstract: On the basis of the experimental data of phase equilibria and thermochemical properties available from literatures, a critical assessment for the Ni-Yb binary system was carried out using the CALPHAD (calculation of phase diagrams) method. The liquid phase is modeled as the associate model with the constituent species Ni, Yb and YbNi3, owing to the sharp change of the enthalpy of mixing of liquid phase at the composition of around 25% Yb (mole fraction). The terminal solid solutions FCC_A1 (Ni/Yb) and BCC_A2 (Yb) are described by the substitutional solution model with the Redlich-Kister polynomial. The intermetallic compounds, Yb2Ni17, YbNi5, YbNi3, YbNi2, α-YbNi and β-YbNi, are treated as strict stoichiometric compounds, since there are no noticeable homogeneity ranges reported for these compounds. A set of self-consistent thermodynamic parameters for the Ni-Yb binary system are obtained. According to the presently assessed results, the thermochemical properties and the phase boundary data can be well reproduced.


