Trans. Nonferrous Met. Soc. China 23(2013) 3068-3075
Leaching of heavy metals from Dexing copper mine tailings pond
Yao-guang GUO, Peng HUANG, Wu-gang ZHANG, Xue-wu YUAN, Feng-xia FAN, Huan-li WANG, Jian-she LIU, Zhao-hui WANG
State Environmental Protection Engineering Center for Pollution Treatment and Control in Textile Industry, College of Environmental Science and Engineering, Donghua University, Shanghai 201620, China
Received 8 October 2012; accepted 21 December 2012
Abstract:
The wastewater source of 4# tailing pond in Dexing copper mine consists of alkaline flotation pulp and acid mine drainage (AMD) from the nearby opencast mine. Therefore, the heavy metals in tailing ore are very likely to be released due to acidification from AMD. The leaching behaviors of Zn, Cu, Fe and Mn in mine tailings from Dexing copper mine were investigated by a series of laboratory batch experiments. The effectcs of pH, temperature, particle size and contact time on the leachability of such heavy metals were examined. It was evident that Zn, Cu, Fe and Mn were major heavy metals in the tailings while gangue minerals like quartz were major constituents in examined tailings. The tailing dissolution reaction was controlled by the acid, whose kinetics could be expressed according to the heterogeneous reaction models and explained by a shrinking core model with the surface chemical reaction as the rate-controlling step. The leachability of all metals examined depended on pH and contact time. The batch studies indicated that the maximum leaching ratios of Zn, Cu, Fe and Mn at pH 2.0 were 5.4%, 5.8%, 11.1% and 34.1%, respectively. The dissolubility of all metals examined was positively correlated to the temperatures. The particle size would not change dissolution tendency of those heavy metals, but decrease the concentrations of leached heavy metals.
Key words:
leaching; tailings; heavy metals; dissolution rate;
1 Introduction
Dexing copper mine, covering an area of about 100 km2 and at an altitude range of 65-500 m, is located in the Sizhou town (latitude/longitude: 29°43′ N/117°02′ E) of Dexing city, Jiangxi province, China. The 4# tailing pond (14.3 km2 area) with a design capacity of 8.35×109 m3, is still currently in use, where approximately 1×105 t of flotation tailings are deposited per day [1]. Therefore, the 4 # tailing reservoir has become the biggest one in Asia.
According to incomplete statistics, there are more than 4×104 t of acid mine drainage(AMD) and 3.32×105 t of alkaline flotation wastewater discharged out of Dexing copper mine each day, while about 1.3×104 t of AMD are poured into the 4# tailing pool [2]. Nevertheless, the amount of AMD is closely related to rain season, which is rich in summer but poor in winter. The fluctuation in quantity of AMD could lead to pH change, thereby significantly influencing the leachability of heave metals [3,4]. Al-ABED et al [5] indicated that arsenic leaching was of strong dependence on pH in batch leaching tests at different pH conditions, i.e. arsenic leaching followed a ‘‘V’’ shaped profiles with significant leaching both in the acidic and alkaline pH regions. Moreover, it is significant to reduce the risk of metal uptake toxicity by plants and animals when a neutral pH is maintained in the tailings at the time of the plant establishment [6], and the low pH may lead to increasing solubility and thus higher plant uptake of the heave metals in the tailings instead [7]. When potentially toxic metal elements accumulated in environment, they can induce a potential contamination of food chain and endanger the ecosystem safety and human health [8-14]. Importantly, the heavy metals have a negative influence on post-wastewater reuse [15]. For instance, heavy metal ions can result in deterioration of flotation selectivity [16].
In general, release of heavy metals is considered to be caused by proton-induced mineral dissolution in which some acidophilic iron-oxidizing bacteria such as Acidithiobacillus ferrooxidans, A. thiooxidans, Leptospirillum ferrooxidans, L. ferriphilum, play a dominant role [17-21]. These microorganisms are capable of oxidizing iron and sulfur in sulfide ore tailings, thereby resulting in the mineral dissolution and metals leaching. However, the special conditions in the selected 4# tailing pond make the acidophilic iron-oxidizing bacteria hardly survive since alkaline flotation wastewater is also discharged into the this tailing pond. Consequently, it is convinced that the process of leaching of heavy metals from the tailings was mainly abiotic process. The study on the release of heavy metals induced by acidification from Dexing tailings has not yet reported currently.
Hence, the objective of the present study is to discuss the effects of pH, temperature, particle size and contact time on the release of heavy metals in 4# tailing reservoir of Dexing copper mine, and then provide useful information for better understanding the risk of heavy metals release to ambient aquatic environment and mine wastewater reuse.
2 Experimental
2.1 Experimental tailings
Samples were collected from non-acidified area in Dexing copper 4# tailing reservoir. Air-dried mine tailings were passed through sieve with 150 μm in sieve size for homogenization to remove large particles. In order to investigate the effect of particle size on the leachability of heavy metals from tailing samples, the grained particles with diameter less than 1700 μm were also used.
2.2 Batch tests
A range of pH between 2.0 and 7.0 (different series of experiments for initial pH 2.0, 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0 and 7.0) was selected to check the leachability of heavy metals under acidic and neutral conditions. For each leaching experiment, 25 g of tailings and 250 mL acid solution were placed in 250 mL glass beaker sealed with plastic film. An adjustment was taken so as to receive the appropriate pH values. The leachate was collected every five days, and analyzed for heavy metal concentrations. The experiments were conducted at room temperature in 50 d duration.
2.3 Analytical methods
Metal concentrations (Cu, Zn, Cd, Cr, Fe, Ca, Mn, Al, Pb, Mg) in the tailings were determined by acid digestion (HNO3/HClO4/HF/HCl) with analysis using an inductively coupled plasma-atomic emission spectrometry (ICP-AES, IRIS Intrepid, Thermo Electron Corporation, CA). Metal concentrations of leachate were analyzed using a atomic absorption spectrometer (AAS). X-ray diffraction (XRD) patterns were measured on a Philips MPD 18801 X-ray diffractometer equipped with Cu Kα radiation. X-ray photoelectron spectroscopic (XPS) measurement was performed on a 2201-XL multi- functional spectrometer (VG Scientific England) using Al Kα radiation.
3 Results and discussion
3.1 Characterization of tailings sample
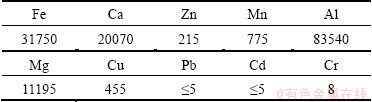
The results of elemental analysis of the tailings are summarized in Table 1. The results show that the tailing samples contained at least 10 different metals, and were rich in heavy metals such as Fe, then followed by Mn, Cu, Zn and Cr. The copper content exceeded class III of environmental quality standard for soils. In the present study, the leaching behaviors of Fe, Mn, Cu and Zn were examined due to their abundances in tailings.
Table 1 Total contents of metals in tailings sample (mg/kg)
The XRD pattern of the original tailing sample is shown in Fig. 1. XRD data were collected in the angular range (10°-90°). The tailings mainly consist of quartz, despujolsite and muscovite-3T, with trace palygorskite O and pectolite, manganoan. Nevertheless, not all their characteristic peaks for Fe and Cu oxides were identified although they were abundant in tailings as-evidenced by elements analysis, possibly due to the interference and coverage with abundant Ca, Al and Si oxides, or their occurrences in the non-crystalline structure.
3.2 Kinetic analysis
When the tailings were added into the hydrochloric acid solution, the main reactions were dissolution reactions controlled by the acid. Taking despujolsite (Ca3Mn(SO4)2(OH)6(H2O)3), one main component of the tailings, for example, the reactions taking place in the hydrochloric acid solution can be written as follows:
6HCl(aq)=6H+(aq)+6Cl-(aq) (1)
Ca3Mn(SO4)2(OH)6(H2O)3(s)+6H+(aq)=3Ca2+(aq)+Mn4+(aq)+ +9H2O(l) (2)
+9H2O(l) (2)
The overall reactions can be written as follows:
Ca3Mn(SO4)2(OH)6(H2O)3(s)+6HCl(aq)=3Ca2+(aq)+Mn4+(aq)+ +6Cl-(aq)+9H2O(l) (3)
+6Cl-(aq)+9H2O(l) (3)
The rate of the tailings dissolution reaction controlled by the acid could be expressed according to the heterogeneous reaction models. In the model, it was considered that the reaction could occur on the outer surface of the unreacted core of particle. The unreacted core of the particle shrank and the layer of the solid product thickened with the reaction proceeding. As no ash was formed, the reacting solids shrank during the reaction, finally disappeared. According to the model, three steps summarized below were considered to take place in succession during reaction [22]:
1) External diffusion of the fluid reactant through the fluid film surrounding the particle to the solid surface;
2) Fluid-solid chemical reaction on the surface between the fluid reactant and the solid;
3) Diffusion of the reaction products from the solid surface through the fluid film back into the bulk fluid solution. Note that the ash layer was in absence and showed no resistance.
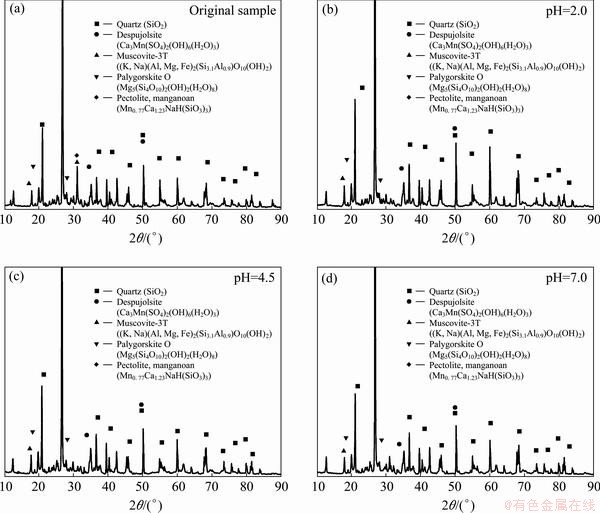
Fig. 1 XRD patterns of tailings samples before (a) and after leaching at pH 2(b), 4.5(c) and 7.0(d)
The concentration of the fluid phase was considered to be unchanged during reaction, in any case where its concentration was high. For such situations in which the concentration of fluid reactant was constant, the integrated rate equations were given as follows.
If the process is controlled by the film diffusion of fluid, Eq. (4) is used:
 (4)
(4)
If this is controlled by resistance of chemical surface reactions, Eq. (5) is used:
 (5)
(5)
where XB is the conversion rate of B, t is the time for conversion; ρB is the molar density of solid reactant; R0 is the radius of the solid sphere; b is the stoichiometric coefficient of the solid; kl is the liquid diffusion coefficient; ks is the solid diffusion coefficient; CA is the concentration of A in the bulk solution.
The dissolution kinetics of same metal oxides or metal hydroxide minerals in acid solutions, such as natural magnesite, colemanite, ulexite, had proved that the dissolution rate was controlled by the surface reaction [23-26]. The leaching rate for certain mineral was strongly related to the acid concentration (i.e. pH), temperature and particle size, since these influencing factors directly affect the parameters like CA, kl, R0 in Eq. (5), thereby exerting influence over the conversion rate XB.
3.3 Effect of pH
Figure 2 represents the results for leaching behaviors of Zn, Cu, Fe and Mn at different pH. It could be seen that the leachability of all metals examined depends on pH and contact time. Leachate has higher metal concentrations at low pH such as 2.0, whereas at neutral pH concentrations of four heavy metals are nearly nondetectable. With the increase of contact time, concentrations of four metal ions in leachate increase, reach maximum values and then decrease to nondetectable values, which had been reported in Refs. [27-29]. The peaks in concentrations of four heavy metals possibly represent the limited fractions of easily dissolved metals in the mine tailings.
The decay duration is found to be closely related to initial pH. For example, Fe concentration in leachate at pH 2.0 at the 25th day just attains the maximum whereas it has been hardly detected at pH 4.5-7.0 at the same time (25 d). These fluctuations in metal concentrations should be ascribed to metals hydrolysis and precipitation processes at higher pH. These experimental results have significant implications for management of tailing pond and water reuse. It suggests that the risk of heavy metal release is the largest at acidic pH and the contact time of 15-20 d when floatation slurry and acid mine drainage are mixed. In this case, a longer hydraulic retention time for wastewater in tailings pond should be beneficial for its reuse for flotation.
Figure 3 shows the maximum extents of dissolution of heavy metals at pH 2.0-7.0. Under acidic conditions, the higher amounts of leached Zn, Cu, Fe and Mn are found compared with those at higher pH. The results of the batch studies exhibit that the maximum leaching rates of Zn, Cu, Fe and Mn are 5.4%, 5.8%, 11.1% and 34.1%, respectively. These results are in accordance with those reported recently in Refs. [30,31]. They also found that Mn was easily leached from slag. XRD results indicate that Mn might be dissolved into solutions since the characteristic peak of Mn-bearing mineral Mn0.77Ca1.23NaH(SiO3)3 at pH 2.0-7.0 is nearly not observed in Fig. 1. XPS spectrum (data not shown) shows that Fe 2p3/2 peak in original sample is observed at 710.9 eV while that of the leached sample at pH 2.0 is shifted to 711.5 eV, indicating that the chemical states of the iron species in tailing samples change after acid dissolution. Mole fraction of iron element in leached sample at pH 2.0 decreases from 1.02% (original) to 0.77%.
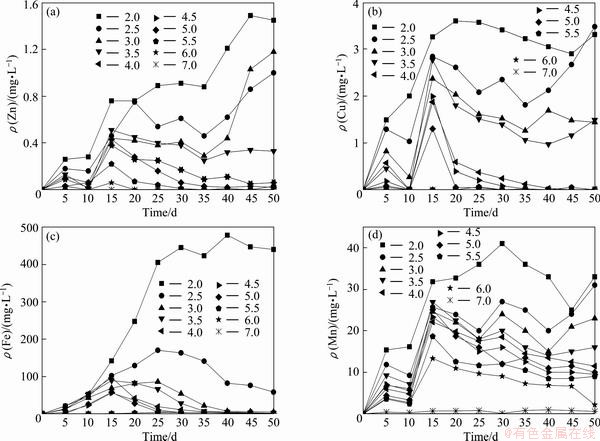
Fig. 2 Total concentrations of Zn (a), Cu (b), Fe (c) and Mn (d) in leachate at different pH during 50 d of leaching processes
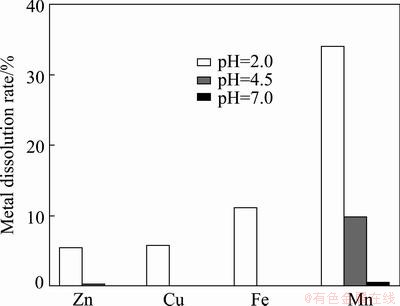
Fig. 3 Maximum dissolution rates of heavy metals at different pH
3.4 Effect of temperature
The effect of temperature on metals leaching properties is exhibited in Fig. 4. The leachability tendency of those heavy metals would hardly be changed at different temperatures. However, those heavy metals levels decline slightly at lower temperatures. The concentrations of all metals examined in leachate at lower temperatures are considerably less than those at higher temperatures. This leaching behavior could be well understood from the following five equations. With the temperature increment, the solubility product constant Ksp increases, which can be seen from Eq. (10). Thus, the leaching concentrations of those heavy metals would be increased (Eq. (7)). Hence, the measured concentrations of heavy metals in the leachate would represent a maximum of the leaching level under the same conditions, when higher temperature was examined in the study herein.
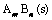

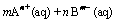 (6)
(6)
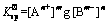 (7)
(7)
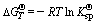 (8)
(8)
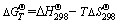 (9)
(9)
 (10)
(10)
3.5 Effect of tailings particle size
Figure 5 shows the dependence of metals leaching properties on particle size. The particle size would not change dissolution tendency of those heavy metals, but peak concentrations and heavy metals levels both decrease slightly. Metal concentrations in the leachate of large sized particles are considerably less than those of fine particles. This leaching behavior could be well understood because fine-grained particles have larger surface area in contact with the solution than coarse particles. Therefore, the measured concentrations of heavy metals in the leachate would represent a maximum of the leaching level under the same conditions, when fine grained particles (<150 μm) were examined in the current study.
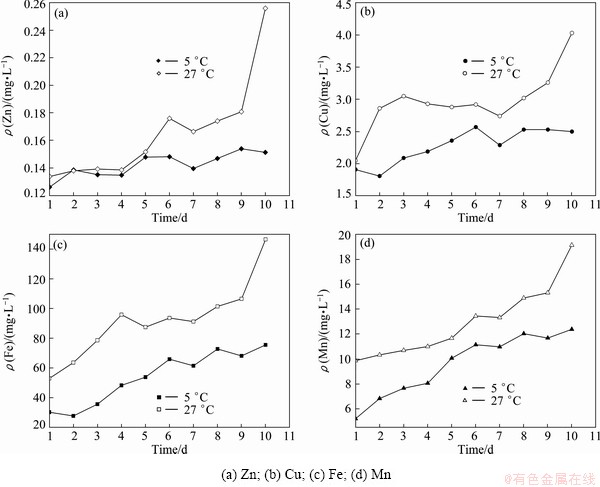
Fig. 4 Effect of temperature on release of heavy metals at pH=2.0
Fig. 5 Effect of particle size of tailings on release of heavy metals at pH=2.0
4 Conclusions
1) The tailing samples of 4# tailing reservoir in Dexing copper mine contain large amount of heavy metals such as Fe, Mn, Cu and Zn, posing a potential threat to aquatic environment and its wastewater reuse. Gangue minerals like quartz are major components in examined tailings.
2) The rate of the tailings dissolution reaction controlled by the acid could be expressed according to the heterogeneous reaction models and it obeys a shrinking core model with the surface chemical reaction as the rate-controlling step.
3) The leachability of all metals examined depends on pH and contact time. Their dissolved metals concentrations firstly increase and then decline after attaining the maximum values. The decay duration is found to be closely related to initial pH. The batch studies exhibit that the maximum leaching ratios of Zn, Cu, Fe and Mn at pH 2.0 are 5.4%, 5.8%, 11.1% and 34.1%, respectively.
4) The dissolubility of all metals in the tailings examined is pretty affected by the temperatures. The samples at higher temperatures lead to higher leaching concentrations than at lower temperatures.
5) The solubility characteristics of tailings are slightly influenced by particle sizes. The dissolution tendency of those heavy metals could hardly be changed by the particle size, both peak concentrations and heavy metals levels only decrease to some degree.
References
[1] XIE Xue-hui, FU Jin, WANG Hui-ping, LIU Jian-she. Heavy metal resistance by two bacteria strains isolated from a copper mine tailing in China [J]. African Journal of Biotechnology, 2010, 9(26): 4056-4066.
[2] YAN Rong-qing. Optimization of the control system for acid drainage treatment in Dexing copper mine [J]. Metal Mine, 2009(8): 130-131. (in Chinese)
[3] RAO Yun-zhang, HOU Yun-bing. Study on law of acidification of wastewater in tailings reservoir and heavy metals pollution [J]. Journal of Liaoning Technical University, 2004, 23(3): 430-432. (in Chinese)
[4] AELION C M, DAVIS H T, MCDERMOTT S, LAWSON A B. Metal concentrations in rural topsoil in south Carolina: Potential for human health impact [J]. Science of the Total Environment, 2008, 402: 149-156.
[5] AL-ABED S R, JEGADEESAN G, PURANDARE J, ALLEN D. Arsenic release from iron rich mineral processing waste: Influence of pH and redox potential [J]. Chemosphere, 2007, 66: 775-782.
[6] FORSBERG L S, LEDIN S. Effects of sewage sludge on pH and plant availability of metals in oxidising sulphide mine tailings [J]. Science of the Total Environment, 2006, 358: 21-35.
[7] ROSS S M. Retention, transformation and mobility of toxic metals in soils [C]//ROSS S M. Toxic Metals in Soil-Plant Systems. Surrey: John Wiley and Sons, 1994: 63: 152.
[8] REYNDERS H, BERVOETS L, GELDERS M, de COEN W M, BLUST R. Accumulation and effects of metals in caged carp and resident roach along a metal pollution gradient [J]. Science of the Total Environment, 2008, 391: 82-95.
[9] SHAH K, NONGKYNRIH J M. Metal hyperaccumulation and bioremediation [J]. Biologia Plantarum, 2007, 51(4): 618-634.
[10] LI Wei-xin, ZHANG Xu-xiang, WU Bing, SUN Shi-lei, CHEN Yan-song, PAN Wen-yang, ZHAO Da-yong, CHENG Shu-pei. A comparative analysis of environmental quality assessment methods for heavy metal-contaminated soils [J]. Pedosphere, 2008, 18(3): 344-352.
[11] TENG Yan-guo, NI Shi-jun, WANG Jin-sheng, ZUO Rui, YANG Jie. A geochemical survey of trace elements in agricultural and non-agricultural topsoil in Dexing area [J]. Journal of Geochemical Exploration, 2010, 104(3): 118-127.
[12] 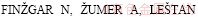 D. Heap leaching of Cu contaminated soil with [S,S]-EDDS in a closed process loop [J]. Journal of Hazardous Materials B, 2006, 135: 418-422.
D. Heap leaching of Cu contaminated soil with [S,S]-EDDS in a closed process loop [J]. Journal of Hazardous Materials B, 2006, 135: 418-422.
[13] VEGA F A, COVELO E F, ANDRADE M L, MARCET P. Relationships between heavy metals content and soil properties in mine soils [J]. Analitica Chimica Acta, 2004, 524: 141-150.
[14] LEI Liang-qi, SONG Ci-an, XIE Xiang-li, LI Yan-hong, WANG Fei. Acid mine drainage and heavy metal contamination in groundwater of metal sulfide mine at arid territory (BS mine, Western Australia) [J]. Transactions of Nonferrous Metals Society of China, 2010, 20: 1488-1493.
[15] MONCUR M C, PTACEK C J, BLOWES D W, JAMBOR J L. Release, transport and attenuation of metals from an old tailings impoundment [J]. Applied Geochemistry, 2005, 20: 639-659.
[16] DAI Zi-lin, JIANG Qing-mei, CHEN Zhi-qiang, FENG Qi-ming. Study on the flotation process for complex Cu-Pb-Zn sulphide ore [J]. Materials Research and Application, 2008, 2(3): 234-237. ( in Chinese)
[17] SHI Shao-yuan, FANG Zhao-heng, NI Jin-ren. Comparative study on the bioleaching of zinc sulphides [J]. Process Biochemistry, 2006, 41: 438-446.
[18] LIU Yun-guo, ZHOU Ming, ZENG Guang-ming, LI Xin, XU Wei-hua, FAN Ting. Effect of solids concentration on removal of heavy metals from mine tailings via bioleaching [J]. Journal of Hazardous Materials, 2007, 141: 202-208.
[19] LIU Yun-guo, XIAO Xin, LI Xin, TIAN Da-lun, ZENG Guang-ming, RAO Yuan-hong. Bioleaching of heavy metals from mine tailings by indigenous microorganisms [J]. Journal of Hunan University: Natural Sciences, 2011, 38(1): 70-74. (in Chinese)
[20] DONG Ying-bo, LIN Hai, FU Kai-bin, MU Xiao-lan, WANG Han. Effect of chemical mutation on the microbial leaching of low grade copper tailings [J]. Journal of University of Science and Technology Beijing, 2011, 33(5): 532-538. (in Chinese)
[21] KONDRAT'EVA T F, PIVOVAROVA T A, BULAEV A G, MELAMUD V S, MURAVYOV M I, USOLTSEV A V, VASIL'EV E A. Percolation bioleaching of copper and zinc and gold recovery from flotation tailings of the sulfide complex ores of the Ural region, Russia [J]. Hydrometallurgy, 2012, 111-112: 82-86.
[22] MAZET N. Modeling of gas-solid reactions.1. Nonporous solids [J]. International Chemical Engineering, 1992, 32: 271-284.
[23] BAYRAK B, 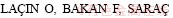 H. Investigation of dissolution kinetics of natural magnesite in gluconic acid solutions [J]. Chemical Engineering Journal, 2006, 117: 109-115.
H. Investigation of dissolution kinetics of natural magnesite in gluconic acid solutions [J]. Chemical Engineering Journal, 2006, 117: 109-115.
[24] ALKAN M, DOGAN M. Dissolution kinetics of colemanite in oxalic acid solutions [J]. Chemical Engineering and Processing, 2004, 43: 867-872.
[25] 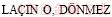 B, DEMIR F. Dissolution kinetics of natural magnesite in acetic acid solutions [J]. International Journal of Mineral Processing, 2005, 75: 91-99.
B, DEMIR F. Dissolution kinetics of natural magnesite in acetic acid solutions [J]. International Journal of Mineral Processing, 2005, 75: 91-99.
[26] EKMEKYAPAR A, DEMIRKIRAN N, 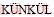 A. Dissolution kinetics of ulexite in acetic acid solutions [J]. Chemical Engineering Research and Design, 2008, 86: 1011-1016.
A. Dissolution kinetics of ulexite in acetic acid solutions [J]. Chemical Engineering Research and Design, 2008, 86: 1011-1016.
[27] SCHWAB P, ZHU D, BANKS M K. Heavy metal leaching from mine tailings as affected by organic amendments [J]. Bioresource Technology, 2007, 98: 2935-2941.
[28] CAO Yun-quan, ZHANG Shuang-sheng, LIU Han-hu, LIU Wei. Leaching characteristics of heavy metals in coal gangue in both dynamic leaching and static immersion [J]. Journal of Hebei University of Engineering, 2010, 27(1): 76-80. (in Chinese)
[29] JING Chuan-yong, MENG Xiao-guang, GEORGE P K. Lead leachability in stabilized/solidified soil samples evaluated with different leaching tests [J]. Journal of Hazardous Materials B, 2004, 114: 101-110.
[30] AL-ABED S R, HAGEMAN P L, JEGADEESAN G, NARAIN M, ALLEN D. Comparative evaluation of short-term leach tests for heavy metal release from mineral processing waste [J]. Science of the Total Environment, 2006, 364: 14-23.
[31] MOUSTAKAS K, MAVROPOULOS A, KATSOU E, HARALAMBOUS K J, LOIZIDOU M. Leaching properties of slag generated by a gasification/vitrification unit: The role of pH, particle size, contact time and cooling method used [J]. Journal of Hazardous Materials, 2012, 207-208: 44-50.
德兴铜矿尾矿库重金属的浸出
郭耀广, 黄 鹏, 张武刚, 袁学武, 范凤霞, 王焕丽, 柳建设, 王兆慧
东华大学 环境科学与工程学院,国家环境保护纺织工业污染防治工程技术中心,上海 201620
摘 要:德兴铜矿的4#尾矿库废水来源包括碱性浮选矿浆和附近露天矿山的酸性废水(AMD)。因此,酸性矿山废水的酸化作用极可能造成尾矿中重金属的浸出。通过实验室的批次实验对德兴铜矿尾矿中锌、铜、铁和锰的浸出行为进行了研究,对pH值、温度、颗粒大小和接触时间对重金属浸出的影响进行了讨论。结果表明,锌、铜、铁和锰是尾矿中的主要重金属,石英类脉石矿物是尾矿的主要成分。尾矿的溶解反应受酸度控制,其动力学可以根据非均相反应的模型来表达,通过表面化学反应作为速率决定步骤的核收缩模型来解释。这些重金属的浸出依赖于pH值和接触时间。批次实验研究得出,锌、铜、铁和锰在pH= 2.0时的最大浸出率分别为5.4%、5.8%、11.1% 和 34.1%。重金属的溶出与温度呈正相关性。颗粒大小不会改变这些重金属的浸出趋势,但会略微导致重金属的最高浓度和平均水平的下降。
关键词:浸出;尾矿;重金属;溶解速率
(Edited by Hua YANG)
Foundation item: Projects (41073060, 21007009) supported by the National Natural Science Foundation of China; “Chen Guang” project (10CG34) supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation, China; Projects (20100075120010, 20100075110010) supported by Research Fund for the Doctoral Program of Higher Education of China
Corresponding author: Jian-she LIU; Tel: +86-21-67792523; E-mail: liujianshe@dhu.edu.cn; Zhao-hui WANG; Tel: +86-21-67792557; E-mail: zhaohuiwang@dhu.edu.cn
DOI: 10.1016/S1003-6326(13)62835-6
Abstract: The wastewater source of 4# tailing pond in Dexing copper mine consists of alkaline flotation pulp and acid mine drainage (AMD) from the nearby opencast mine. Therefore, the heavy metals in tailing ore are very likely to be released due to acidification from AMD. The leaching behaviors of Zn, Cu, Fe and Mn in mine tailings from Dexing copper mine were investigated by a series of laboratory batch experiments. The effectcs of pH, temperature, particle size and contact time on the leachability of such heavy metals were examined. It was evident that Zn, Cu, Fe and Mn were major heavy metals in the tailings while gangue minerals like quartz were major constituents in examined tailings. The tailing dissolution reaction was controlled by the acid, whose kinetics could be expressed according to the heterogeneous reaction models and explained by a shrinking core model with the surface chemical reaction as the rate-controlling step. The leachability of all metals examined depended on pH and contact time. The batch studies indicated that the maximum leaching ratios of Zn, Cu, Fe and Mn at pH 2.0 were 5.4%, 5.8%, 11.1% and 34.1%, respectively. The dissolubility of all metals examined was positively correlated to the temperatures. The particle size would not change dissolution tendency of those heavy metals, but decrease the concentrations of leached heavy metals.


