J. Cent. South Univ. Technol. (2007)05-0656-04
DOI: 10.1007/s11771-007-0126-9 ![]()
Technical optimization of LiFePO4 preparation by water quenching treatment
PENG Zhong-dong(彭忠东), GAO Xu-guang(高旭光), HU Guo-rong(胡国荣),
TAN Xian-yan(谭显艳), DU Ke(杜 柯), DENG Xin-rong(邓新荣), LIU Ye-xiang(刘业翔)
(School of Metallurgical Science and Engineering, Central South University, Changsha 410083, China)
Abstract:
A technique of combination of vacuum firing and water quenching was applied to the synthesis of LiFePO4 powder. The sample was prepared by heating the pre-decomposed precursor mixtures sealed in vacuum quartz-tube, followed by water quenching at the sintering temperature. The synthetic conditions were optimized by orthogonal experiment. The results indicate that the fast quenching treatment can avoid the overgrowth of single crystal and improve its availability ratio of active material. The sintering temperature has the greatest effect on the electrochemical performance of sample. Next is the molar ratio of Li to Fe and the sintering time, respectively. The samples prepared in the optimized technical condition has the highest reversible discharge specific capacity of 149.8 mA?h/g.
Key words:
Li-ion batteries; LiFePO4; cathode material; water quenching ;
1 Introduction
Since last decade, lithium ion batteries have been widely used in devices such as cellular phone and notebook-type computers due to high energy density, perfect cyclic performance and excellent capacity retention[1]. As far as now, the main materials used as cathodes in lithium ion batteries are transitional metal oxides, including layered lithium cobalt oxide and spinel lithium manganese oxide. Lithium cobalt oxide is the most successful commercial material in small size batteries, but it is limited by cobalt resources scarcity and its unsafety during overcharge. Spinel lithium manganese is utilized only in narrow field for its low specific capacity and poor cycling performance, especially at higher temperature (55 ℃)[2-3].
During the last few years, orthorhombic olivine compound LiFePO4 has drawn considerable attention for its application as a cathode material for lithium ion batteries[4-5]. This compound has a theoretical capacity of 170 mA?h/g and is environmentally benign[4-5]. In addition, compared with other cathode oxide materials, such as LiCoO2 and LiNi1-x-yCoxMnyO2, LiFePO4 possesses good thermal stability and is suitable for large-scale rechargeable Li-ion battery applications[2].
However, the key barrier limiting its application is low electronic conductivity of about 10-8-10-9 S/cm at room temperature[4,6]. The electronic conductivity of LiFePO4 can be improved via various materials processing methods, such as coating particles with conductive carbons[7-9], doping with supervalent metal ions[6,10], and synthesis of nanoparticles[11-13]. By far, the most effective way is to coat carbon by adding organic or polymeric precursors during synthesis. However, the reduction of its tap density cannot be avoided by carbon modification, even if the carbon content in final sample is 1.0% (mass fraction)[14]. To maximize power capability without incurring an energy density penalty, it is therefore necessary to optimize the synthetic conditions in the absence of carbon. Obviously, the modification of minimizing the particle size of the sample instead of lowering its crystallization degree can be beneficial to the electronic transport and ion diffusivity. The samples with uniform and fine particle size are of a higher availability ratio of active material, which can decrease electrode polarization and increase the contact area between cathode material and electric additive. The behavior of the crystallization and crystal size distribution (CSD) depends on the course of supersaturation during batch crystallizations. In order to get specified crystallization behavior and CSD the operation condition of cooling mode should be studied and optimized. After about four years study, it is found that fast quenching technique is very effective in controlling the overgrowth of grain size and improving its electrochemical performance. In the present work, the synthetic process of LiFePO4 compound was optimized by water quenching treatment in order to prepare the sample with uniform and fine particle, as well as good crystallization degree.
2 Experimental
LiFePO4 samples were prepared by a technique of combination of vacuum firing and water quenching. Starting materials were Li2CO3 (99.95%), FeC2O4?2H2O (99%) and NH4H2PO4 (98%) in the stoichiometric ratio. The experiments were carried out by a four-step treatment. In the first step, powders were heated at 400 ℃ for 6 h. They were then crushed and ground and pressed into a pellet. In the second step, the pellet was sealed into a piece of quartz-tube in vacuum state. In the third step, the vacuum-tight samples were finally calcined in muffle furnace and then quenched in water at the heating temperature. Finally the cathode powder was obtained by breaking the quartz-tube into pieces and separating the sample with the fragile quartz piece after the temperature was cooled down.
The crystalline structures of the prepared LiFePO4 powders were studied by an X-ray diffraction (XRD, D/max-r A type Cu Kα 40 kV, 300 mA, 10?-70?, Japan). Thus the obtained patterns were used to calculate the lattice parameters of the olivine structure.
Scanning electron microscope (SEM, KYKY 2800, Japan) was used to analyze the morphology of the prepared powders.
The prepared LiFePO4 powders were mixed with acetylene carbon black and polyvinylidene fluoride (PVDF) in mass ratio of 8?1?1 in adequate amount of N-methyl-2 pyrrolidinone (NMP) and stirred for 12 h sufficiently. Then the mixtures were tape-cast on aluminum foil and placed in an oven vacuum and dried at 120 ℃ for 8 h to remove the residual solvent. Finally, the dried tape was punched into round plates with diameter of 12.0 mm as the cathode electrodes. Thus prepared cathodes and Celgard 2400 separator were placed into an argon-filled glove box and assembled into the 2025 coin-type cells with lithium anode, electrolyte of 1 mol/L LiPF6 in EC-DEC (1?1, volume ratio), and the other components of the coin-type cell. Charge/discharge characteristics of the active material were recorded at 0.1C rate (15 mA/g) over a voltage range of 2.5-4.1 V using a battery test system (LAND CT2001A).
3 Results and discussion
In order to optimize the synthetic conditions and study their effects on the electrochemical performance of sample, an orthonormal experiment was performed. The results are shown in Table 1.
Table 1 Orthonormal experimental arrangement
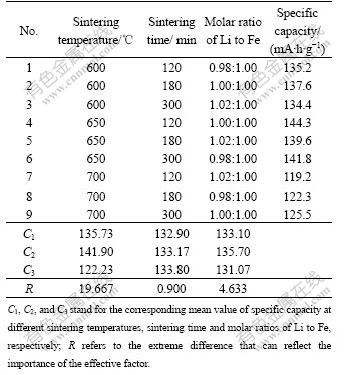
From the extreme difference in Table 1, it can be known that the sintering temperature is the most important factor, the next factor is the molar ratio of Li to Fe (n(Li)/n(Fe)), and the least is the sintering time.
Fig.1 shows the charge-discharge curves of LiFePO4 cathodes synthesized at 600, 650 and 700 ℃ respectively for 8 h and 0.1C rate (15 mA/g). With the increase of sintering temperature from 600 to 700 ℃, the discharge capacity decreases from 126.3 to 110.0 mA?h/g. And the peak value appears at 650 ℃. The reason may be that the high temperature is helpful for the improvement of the crystallization. At the lower temperature of 600 ℃, the sample may consist of some amorphous phase that will lower the content of active material and lead to poor electrochemical performance[15]. At the higher temperature of 700 ℃, the overgrowth of particle of sample cannot be avoided and results in low discharge capacity. It should be noted, however, that faster diffusion of lithium ions across the LiFePO4/FePO4 two-phase boundary is only the factor for improving the electrochemical kinetics of this material[11, 15]. At a moderate temperature of 650 ℃, both good crystallization and fine grain can be achieved. The smaller particle sizes can shorten the path of Li+ diffusion and improve the diffusion velocity of Li+.
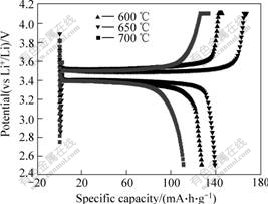
Fig.1 First-cycle voltammograms for LiFePO4 prepared by water quenching way at different sintering temperatures for 8 h and molar ratio of Li to Fe of 1.00?1.00
Fig.2 shows the first-cycle voltammograms of LiFePO4 prepared by water quenching treatment with different molar ratios of Li to Fe, cycled at 0.1C rate. Only the samples with molar ratio of Li to Fe close to 1.00?1.00 have the largest charge-discharge specific capacity. The samples synthesized in this study are different from others because the precursors are sealed in a piece of quartz-tube. The reactive environment belongs to closed system. In this system, the volatile of lithium element can be avoided. The data can be better to reveal the effect of molar ratio of Li to Fe on the electrochemical property of the sample.
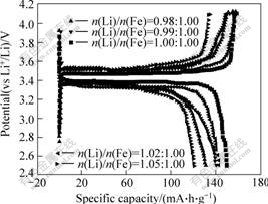
LiFePO4 prepared by water quenching at different molar ratios of Li to Fe and sintered at 650 ℃ for 8 h
Fig.3 displays the first-cycle voltammograms of LiFePO4 cathodes synthesized at 650 ℃ for 10, 120, 180, 300, 480 and 600 min, respectively and 0.1C (15 mA/g) rate. The sample shows relatively low specific capacity of 103.2 mA?h/g at calcining time of 10 min because of low crystallinity. All samples demonstrate high specific capacity over 130 mA?h/g when the calcining time surpass 120 min, especially the sample has the largest specific capacity of 149.8 mA?h/g when the calcining time is 180 min. The reason may be that the appropriate calcining time can guarantee the product with both good crystallinity and fine grain as discussed above. After longer calcining time of more than 180 min, the overgrowth of particle of sample will occur, leading to inaccessibility of part of the active material.
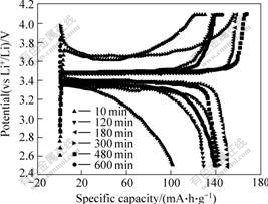
Fig.3 First-cycle voltammograms of LiFePO4 prepared by water quenching method for different sintering times and cycled at 0.1C rate
Fig.4 shows the SEM image of LiFePO4 powders synthesized at 650 ℃ for 180 min. It is found that, the particle size has a slightly micro-aggregate. But the very fine powders with the particle size ranging from 200 and 500 nm are obtained. As it is discussed, the sample with the characteristics of fine particle and good crystallinity may have good electrochemical performance.
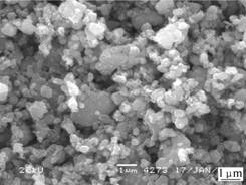
Fig.4 SEM image of LiFePO4 synthesized at 650 ℃ for 180 min and cycled at 0.1 C
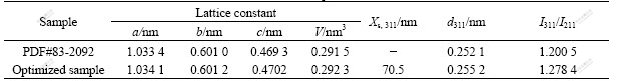
Fig.5 shows the X-ray diffraction pattern of LiFePO4 prepared under optimized conditions molar ratio of Li to Fe of 1.00?1.00 and sintered at 650 ℃ and 180 min. From Fig.5 it can be seen that the sample has perfect olivine structure with sharp diffraction peak and large peak intensity ratio (I311/I211) of 1.278 4 (>1.2). And the lattice constants are a=1.034 1 nm, b=0.601 2 nm, c=0.470 2 nm and V=0.292 3 nm3, which are close to the corresponding constants of PDF value(see Table 2).
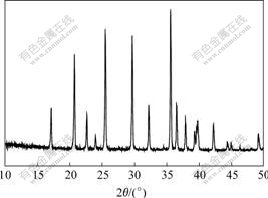
Fig.5 X-ray diffraction pattern of LiFePO4 prepared under optimized conditions of molar ratio of Li to Fe of 1.00?1.00 and sintered at 650 ℃ and 180 min
Table 2 XRD data for optimized LiFePO4
Above all, the optimized sample shows better electrochemical performance and its charge-discharge plateau is very smooth for perfect crystal structure and finer particle size. In the charge process, a flat plateau is quickly formed at 3.5 V, and most of the lithium (0.927 Li/LiFePO4) is extracted in the voltage window to 4.1 V. In the discharge process, the potential rapidly reaches a plateau at 3.4 V, and 0.881Li/LiFePO4, corresponding to a specific capacity of 149.8 mA?h/g, is recovered. The electrochemical performance of the sample is improved greatly by optimizing the technical conditions.
4 Conclusions
1) Water quenching technique was applied to preparing pure LiFePO4 by sintering sample sealed in the vacuous quartz-tube.
2) The technical conditions, including sintering temperature, molar ratio of Li to Fe and calcining time, were studied by orthogonal experiment and the synthetic conditions were optimized. The optimized technical parameters are synthetic temperature of 650 ℃, molar ratio of Li to Fe of 1.00?1.00 and calcining time of 180 min.
3) The sample prepared under optimized conditions has uniform and fine grain size, and considerable crystallinity, which can guarantee better electrochemical performance and kinetics of the two-phase transformation. The specific capacity of the optimized sample reaches 149.8 mA?h/g in discharge process, corresponding to 0.881 mol Li in LiFePO4 reversible.
References
[1] RITCHIE A G.. Recent developments and likely advances in lithium rechargeable batteries[J]. J Power Sources, 2004, 136(2): 285-289.
[2] WHITTINGHAM M S. Lithium batteries and cathode materials[J]. Chem Rev, 2004, 104: 4271-4301.
[3] DAHN J R, FULLER E W, OBROVAC W, et al. Thermal stability of LixCoO2, LixNiO2 and λ-MnO2 and consequences for the safety of Li-ion cells[J]. Solid State Ionics,1994, 69(3/4): 265-270.
[4] PADHI A K, NAJUNDASWAMY K S, GOODENOUGH J B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries[J]. J Electrochem Soc, 1997, 144(4): A1188–A1194.
[5] YANG S, SONG Y, ZAVALIJ P Y, et al. Reactivity, Stability and electrochemical behavior of lithium iron phosphates[J]. Electrochem Comm, 2002, 4(3): 239–244.
[6] CHUNG S Y, BLOCKING J T, CHIANG Y M. Electronically conductive phospho-olivines as lithium storage electrodes[J]. Nature Mater, 2002, 2: 123-128.
[7] SONG M S, KANG Y M, KIM J H, et al. Simple and fast synthesis of LiFePO4/C composite for lithium rechargeable batteries by ball-milling and microwave heating[J]. J Power Sources, 2007, 166(1): 260-265.
[8] XU Zhi-hui, XU Liang, LAI Qiong-yu, et al. Microemulsion synthesis of LiFePO4/C and its electrochemical properties as cathode materials for lithium-ion cells[J]. Materials Chemistry and Physics, 2007, 105(1): 80-85.
[9] HUANG Xiao-wen, SHI Peng-fei. Studies on electrochemical property of LiFePO4/C as cathode material for lithium ion batteries[J]. Chemical Research in Chinese Universities, 2006, 22(1): 73-75.
[10] MOLENDA J, DJCZYK W, SWIERCZEK K, et al. Diffusional mechanism of deintercalation in LiFe1-yMnyPO4 cathode material[J]. Solid State Ionics, 2006, 177(26/32): 2617-2624.
[11] RAVET N, ABOUIMRANE A, ARMAND M, et al. On the electronic conductivity of phospho-olivines as lithium storage electrodes[J]. Nature Mater, 2003, 2: 702-720.
[12] Meligrana G, Gerbaldi C, TUEL A, et al. Hydrothermal synthesis of high surface LiFePO4 powders as cathode for Li-ion cells[J]. J Power Sources, 2006,160(1): 516-522.
[13] YANG M R, KE W H, WU S H. Preparation of LiFePO4 powders by co-precipitation[J]. J Power Sources, 2005, 146(1/2): 539-543.
[14] CHEN Zhao-hui, DAHN J R. Reducing carbon in LiFePO4/C composite electrodes to maximize specific energy, volumetric energy, and tap density[J]. J Electrochem Soc, 2002, 149(9): A1184-A1189.
[15] YAMADA A, CHUNG S C, HINOKUMA K. Optimized LiFePO4 for lithium battery cathodes[J]. J Electrochem Soc, 2001, 148(3): A224–A229.
Foundation item: Project(50604018) supported by the National Natural Science Foundation of China
Received date: 2007-03-10; Accepted date: 2007-05-15
Corresponding author: GAO Xu-guang, Doctoral candidate; Tel:+86-731-8830474; E-mail: csugaoshou@hotmail.com
(Edited by CHEN Wei-ping)
Abstract: A technique of combination of vacuum firing and water quenching was applied to the synthesis of LiFePO4 powder. The sample was prepared by heating the pre-decomposed precursor mixtures sealed in vacuum quartz-tube, followed by water quenching at the sintering temperature. The synthetic conditions were optimized by orthogonal experiment. The results indicate that the fast quenching treatment can avoid the overgrowth of single crystal and improve its availability ratio of active material. The sintering temperature has the greatest effect on the electrochemical performance of sample. Next is the molar ratio of Li to Fe and the sintering time, respectively. The samples prepared in the optimized technical condition has the highest reversible discharge specific capacity of 149.8 mA?h/g.


