Trans. Nonferrous Met. Soc. China 26(2016) 1425-1432
Hydrogenation reaction of metallic titanium prepared by molten salt electrolysis
Qi-gang WENG1, Rui-di LI1, Tie-chui YUAN1, Yu-sheng SHI2, Zi-li QIU1, Ming-xiang JIANG1, Yue-hui HE1
1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. State Key Laboratory of Materials Processing and Die & Mould Technology,
Huazhong University of Science and Technology, Wuhan 430074, China
Received 11 December 2015; accepted 4 May 2016
Abstract:
The hydrogenation reaction of electrolyzed titanium, as the first step during hydrogenation-dehydrogenation for the preparation of titanium powder, was studied. The titanium hydride was prepared through the reaction between electrolyzed titanium and hydrogen at different hydrogenation temperatures and different time. The evolutions of hydrogen and oxygen contents, density, hardness and phase composition before and after hydrogenation were characterized under different hydrogenation conditions. The results show that the main phases of titanium hydride were TiH1.924, TiH1.971 and TiH2. Increasing the hydrogenation temperature could not enhance the hydrogen content but increase the oxygen content. The effect of the hydrogenation time on the hydrogen content was not obvious. The optimal parameters of the hydrogenation process were obtained: heating at 400 °C and holding for 2 h, by which the hydrogen content of 3.63% and oxygen content of 0.18% (mass fraction) can be obtained. In addition, the microstructure, orientations and tissues of electrolyzed titanium and titanium hydride were detected.
Key words:
titanium powder; hydrogenation reaction; molten salt electrolysis; hydrogen content ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ; ;;
1 Introduction
Owing to its excellent physical and chemical properties, titanium powder has found its widespread applications in biomaterials [1,2], powder metallurgy (PM) [3], additive manufacturing [4,5], electronic target [6,7], aerospace [8] and pressure hull [9]. The main preparation methods of titanium powder are rotating electrode atomization and hydrogenation- dehydrogenation (HDH). The rotating electrode atomization could produce spherical powder, but its diameter is coarse and the cost is high. The HDH is another important preparation method for titanium powder [10]. During HDH, the sponge titanium reacts with hydrogen and forms the brittle TiHx, which can be crushed into powder easily, and then the TiHx powder transforms into Ti powder by decomposition reaction at a more high temperature [11,12]. HDH is easy to produce fine Ti powder with concentrated particle size compared with rotating electrode atomization. The study on preparation of Ti powder by HDH is thus necessary. The other important index for elevating property of Ti powder is the oxygen content. The high oxygen content is detrimental for the final properties of titanium components produced from as-obtained titanium powder in powder sintering and additive manufacturing [13-16]. For example, the oxygen in titanium powder is apt to decrease the mechanical strength. Moreover, when the titanium powder is applied to 3D printing additive manufacturing, the oxygen in powder can lead to balling phenomenon, which worsens the forming quality. Usually, the raw material used for HDH process is sponge titanium. The oxygen content in sponge titanium is high, which is unfavorable for the powder properties. Recently, the preparation of high purity titanium has considered to be very important in electronic target, high-quality PM part, additive manufacturing.
Molten salt electrolysis (MSE), as an important purification method of titanium, has been highly valued. The MSE uses low purity sponge titanium as anode material. In the anode process of MSE, the impurities with anode potential higher than that of titanium ion tend to be reserved in anode while the titanium will be dissolved in molten salt. In the cathode process of MSE, the impurity ion with cathode potential lower than that of titanium ion will be reserved in molten salt [17,18]. Thus, the MSE can yield high purity metallic titanium and has been widely applied in industry. Therefore, when MSE- prepared titanium is used for the raw material of HDH, the purity of as-obtained titanium powder could be enhanced compared with that with conventional sponge titanium as raw material. However, the electrolyzed titanium exhibits different microstructures and crystallization characterizations, thus, the hydrogenation reaction behavior may be different from conventional HDH using sponge titanium as raw material. As the first step of HDH, hydrogenation of electrolyzed titanium should be studied carefully.
The hydrogenation of raw titanium material is the primary step of preparation of titanium powder by HDH, which largely determines the hydrogen content, oxygen content and contents of other impurities. Hydrogen in solid solution goes into the titanium material at high temperature, and then reacts with titanium over the critical solubility, which makes the non-stoichiometric compounds-TiHx precipitate. The brittle TiHx is the key to determine whether the titanium hydride can be effectively broken down to the appropriate size range during the subsequent ball milling procedure and the effect of oxygen content on the mechanical properties of the final titanium powder products should not be ignored [19]. Existing research showed that oxygen could greatly improve the strength of titanium and titanium alloy, but could also greatly reduce its plasticity, heat resistance, thermal stability and solderability [19,20]. Therefore, controlling the oxygen content of the finally obtained titanium powder is essential. Among other impurity elements, the TiFe phase intermetallic compound formed by Fe and Ti will decrease the pitting corrosion potential and provide the core of the localized corrosion, which is harmful to the corrosion resistance of the titanium [21]. The fatigue properties of titanium products are greatly affected by the common Cl element in sponge titanium [22]. In the traditional process of HDH, TiCl4 is used as raw material for the reduction of sponge titanium. Although the yield of conventional sponge titanium has advantages in terms of cost, the contents of sodium, magnesium and chloride ion in the sponge titanium obtained by the conventional procedure are high. More than half of impurities of the final titanium products are derived from raw materials, which will make the equipment polluted and the welding properties of materials deteriorate during the sintering process.
Therefore, the MSE titanium, as the raw material for hydrogenation reaction, is favorable for decreasing the oxygen content of the final product. Simultaneously, the hydrogenation reaction kinetics may differ from conventional sponger titanium due to the difference of microstructure. Thus, it is necessary to study the hydrogen reaction of electrolyzed titanium. In the present work, the evolution behaviors of element content, phase, microstructure, hardness and density of TiHx were studied and the optimal techniques were studied.
2 Experimental
The high purity titanium prepared by MSE was used as raw material with particle size ranging from 1 to 10 mm. The hydrogenation process was as follows. First, the titanium powder was placed in vacuum furnace and the furnace was pumped below 1 Pa and then 99.999% purity Ar gas was injected into the furnace. The pumping and injecting Ar gas were repeated 3 times to eliminate the oxygen inside. Second, the electrolyzed titanium was heated in the furnace at 350, 400, 450, 500 and 550 °C and then injected H2 with 99.999% purity and incubated for 0.5-8 h. The pressure of H2 in the furnace is 0.13-0.15 MPa. After hydrogenation, the density, H/O contents, and hardness were measured. After being ground into powder and sifted, titanium hydride was taken to analyze its phase. Optical microscope (Leica, MeF3A) and field emission scanning electron microscope (Nova, NanoSEM 230) were used to observe the microstructure of the alloy after mechanical and chemical polishing and chemical etching. The phase compositions of the samples were characterized by X-ray diffraction (XRD) (Rigaku, D/max 2550VB). The hydrogen content and oxygen content of raw material and titanium hydride were analyzed with an oxygen analyzer (LECO, TCH-436). The density of titanium hydride was measured by a micro-hardness detector (MicroMet 5104).
The electrolytic titanium was the product of Guizhou Zunyi Titanium Industry. In this experiment, titanium hydride, which was prepared with high purity hydrogen at different hydrogenation temperatures and hydrogenation time, was used to study the hydrogenation mechanism, microstructure evolution and appropriate hydrogenation process.
3 Results and discussion
3.1 hydrogen and oxygen contents
Figure 1(a) shows the hydrogen and oxygen contents in raw materials and titanium hydride samples which were hydrogenated and heated at different temperatures for 2 h. In TiHx, the range of x value was 0-2. By calculating the highest hydrogen content in TiH2, the hydrogen content of TiHx was equal or lesser than 4% (mass fraction). Under all the conditions, with the temperature increasing above 400 °C, the hydrogen content changed slightly, while the oxygen content increased dramatically. Among the five groups, the lowest oxygen content in the titanium samples was 0.18%, which was obtained at 400 °C.
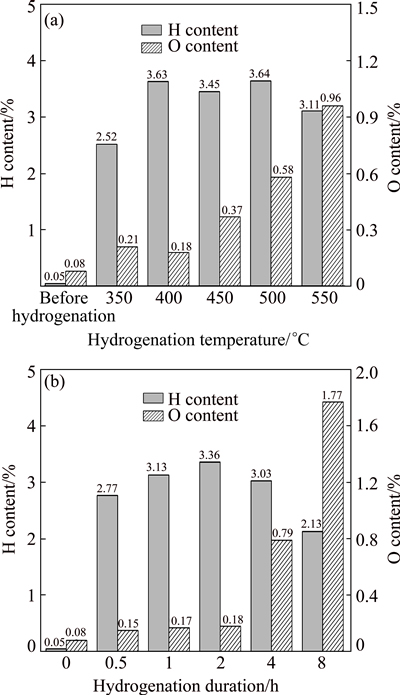
Fig. 1 Hydrogen and oxygen contents in raw materials and titanium hydride samples obtained at different temperatures (a) and different hydrogenation time (b)
Figure 1(b) shows the hydrogen and oxygen contents in raw materials and titanium hydride samples which were hydrogenated and heated for different time at 400 °C. The hydrogen and oxygen contents in the same batches of raw materials were 0.05% and 0.08%, respectively. The lowest hydrogenation time was 0.5 h, and the hydrogen content increased to 2.77%.
Among the five groups, the highest hydrogen content of titanium hydride was 3.36%, which was obtained after 2 h of hydrogenation. The lowest hydrogen content of titanium hydride was 2.13%, which was obtained after 8 h of hydrogenation. The hydrogen contents of titanium hydride which was obtained after 1 h of hydrogenation and 4 h of hydrogenation were 3.13% and 3.03%, respectively. Among the five groups, the lowest oxygen content of the titanium hydride was 0.15%, which was obtained after 0.5 h of hydrogenation, and the highest oxygen content of titanium hydride samples was 1.77%, which was obtained after 8 h of hydrogenation. After 1, 2 and 4 h of hydrogenation, the oxygen contents of titanium hydride samples were 0.17%, 0.18% and 0.79%, respectively.
As shown in the results, the hydrogen content of titanium was significantly improved after hydrogenation even at the lowest hydrogenation temperature and the shortest hydrogenation time. Once the hydrogen went into titanium lattice, regardless of whether hydrogen atoms occupied tetrahedral gap lattice or octahedral interstices of titanium, it led to lattice distortion, which would make lattice constants increase and expansion occur [23].
The dissolution of hydrogen in α-Ti would make the lattice constants increase by 2%-3%. Also, as the β phase is stable element, hydrogen would make the temperature of α-β phase transition reduce. Under normal conditions, the α-β titanium phase transformation occurred at 882 °C. According to the titanium-hydrogen phase diagram, β-Ti could form over 300 °C when hydrogen took part in the reaction. The phase transition resulted in changes in the volume of titanium. Because of the solid solution, the embrittlement of titanium occurred. The decrease of elongation, shrinkage on cross section and toughness made cracks easy to generate during the volume change and opened the hydrogenation channel. Figure 2 shows the morphology of a high pure titanium ingot which was hydrogenated at 500 °C for 2 h. The ingot was absolutely cracked and the flaw grew from the middle of the sample to the border, which showed that the dissolution of hydrogen in titanium increased rapidly since the hydrogenation reaction occurred.

Fig. 2 Cracks on surface of hydrogenated pure titanium cast ingot sample
The hydrogen content in titanium did not increase with rising the hydrogenation temperature. This was thought to because of these the following reasons.
1) The dissolution process of Ti is an exothermic reaction. Under the constant pressure condition, the equilibrium will move in the direction of the decomposition of titanium hydride with increasing the reaction temperature. The reaction formula can be described by following equation:
Ti+H2→TiH2 (ΔHΘ=-144.28 kJ/mol) (1)
As shown in Eq. (1), with increasing the temperature, titanium hydride will be decomposed. The content of hydrogen in the new equilibrium decreased. Thus, the hydrogen content of titanium hydride obtained after hydrogenation at 550 °C was 3.11%, which was lower than those obtained at 400, 450 and 500 °C.
2) Titanium is extremely active. At room temperature, the dense oxide film formed on the titanium surface by the reaction between titanium and oxygen, and this film prevented titanium to be oxidized. The reaction between titanium and oxygen was strong at high temperature. Having a protective effect, the density oxidation film can prevent hydrogen from adsorption and dissociation. With increasing the hydrogenation temperatures, oxygen content increased. The oxidation film of titanium surface reduced the hydrogen dissociation area without hindrance of hydrogen diffusion channels.
The effect of holding time on the hydrogen content was not obvious. However, the oxygen content in samples increased rapidly when hydrogenation time reached 2 h, which may be associated with the ability of the equipment to maintain the vacuum. The gap between the parts of the vacuum mechanical pump might increase due to the long duration of the accumulation of heat. Meanwhile, the viscosity of vacuum pump lubrication might decrease at high temperature, which would reduce the vacuum holding capacity of the equipment. When the hydrogenation time reached 8 h, the oxygen content in sample was 1.77%, which would greatly influence the quality and performance of the TiH2.
3.2 Density of titanium hydride
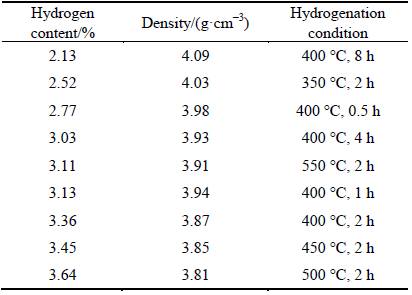
The theoretical density of pure titanium is 4.51 g/cm3, and the density of TiH2 is 3.8 g/cm3. Table 1 shows the hydrogen content and density of titanium hydride obtained under different conditions. As can be seen, with increasing the hydrogen content, the density of the sample decreased gradually.
Table 1 Hydrogen content and density of titanium hydride obtained under different conditions
3.3 Microhardness of raw material and titanium hydride
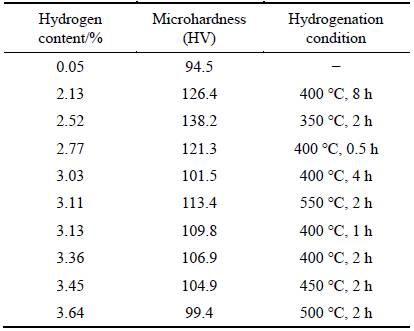
Table 2 shows the hydrogen content and hardness of titanium hydride obtained under different conditions. The hardness of titanium hydride increased compared with that of the raw materials.
The grain size and the precipitation phase in the matrix were important factors to determine the hardness of sample. XU et al [24] reported that the hardness of titanium hydride was about 30% higher than that of pure titanium. After hydrogenation, the volume of material increased. Because of the generation of cracks, the abilities of titanium hydride particles to resist external actions decreased. With improving the hydrogen content, brittle hydride precipitated, which further reduced the mechanical property of titanium hydride and made the titanium hydride more easily to be broken.
Table 2 Hydrogen content and hardness of titanium hydride obtained under different conditions
3.4 X-ray diffraction analysis of titanium hydride
Figure 3(a) shows the XRD patterns of titanium hydride obtained at different temperatures of hydrogenation treatment. All the lines confirmed with standard samples of TiH1.924 and TiH1.971. For TiH2, all the lines shifted about 0.5° to the left compared with standard TiH2 spectral lines. At 400 °C, there was a small peak on the right side of 41.4°. Compared with existing PDF card database, the peak corresponded to TiH2. A similar difference also appeared between 69.7° and 73.9°, and the peak corresponded to the peak of TiH1.924.
Figure 3(b) shows the XRD patterns of titanium hydride obtained with different hydrogenation time at 400 °C. The analysis revealed that all diffraction peaks corresponded to three different titanium hydride phases, TiH1.971, TiH1.924 and TiH2, when the holding time was less than 2 h. When the hydrogenation time reached 4 h, Ti2O and TiO2 phases appeared. Keeping increasing the hydrogenation time, nitride TiN0.3 diffraction peaks appeared.
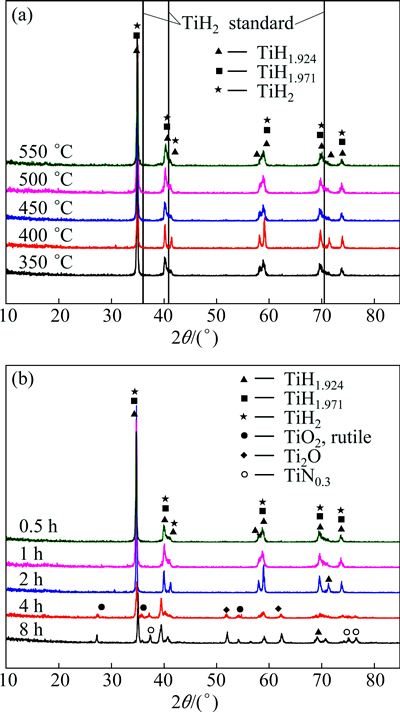
Fig. 3 XRD patterns of titanium hydride obtained at different hydrogenation temperatures (a) and different hydrogenation time (b)
The shift and changes of peaks observed in the XRD patterns were caused by the hydrogen atoms which were dissolved in the titanium matrix. When the solute atoms went into the gaps between crystal lattice, the atomic spacing in the original lattice would change. This was called lattice distortion. With increasing the amount of solute atoms, the degree of lattice distortion increased. Thus, the diffraction peak deviated from the original position, which was reflected in the XRD pattern. Most hydrogen atoms went into titanium and reacted with titanium to form titanium hydride, and others existed in the solid solution. As shown in the XRD patterns, the positions of three vertical lines corresponded to the three strongest peaks of standard TiH2 phase. However, the actual peaks shifted about 0.5° to the left. It showed that lattice distortion happened on hydride, and the left deviation meant that the lattice parameters and crystal surface spacing increased.
3.5 Micromorphologies of raw material and titanium hydride
Figure 4 shows the metallographs of samples before and after hydrogenation. As can be seen from Figs. 4(a) and (b), lath-shaped α-tissue, which spanned about 3-5 μm in width, existed in raw electrolyzed metallic titanium. From the yellow isosceles triangle in Fig. 4(a), these tissues had three specific growth direction at 60° to each other and densely arranged. The number of tissues from the lower left to upper right direction was more than that in the other two directions. After hydrogenation at 400 °C for 2 h, these tissues could not be seen in the obtained titanium hydride and the metallographic figures were uniform. Apparent structure and grain boundaries could not be seen except for some dark holes.
Figure 5 shows the SEM images of samples before and after hydrogenation. As can be seen from Figs. 5(a) and (b), the microstructure was shorter and thicker than that in Figs. 4(a) and (b), and the included angles were not 60° to each other. The yellow circles in Figs. 5(a) and (b) were cross sections of the microstructure which was perpendicular to the surface of the shot. Figures 5(c) and (d) show further amplificatory microstructures of titanium hydride which were obtained after hydrogenation at 400 °C for 2 h. Although the contrast of the picture was not clear enough, the grains could be distinguished.
The major element of the titanium hydride was hydrogen except titanium. Owing to the minimum atomic number, hydrogen could not be measured by the energy spectrum analysis of scanning electron microscopy to know its segregation degree. Because of the differences of the atom arrangement at the grain boundaries and in the crystals, the regular structure of the origin lattice was disturbed by the interface, and the lattice distortion was generated. In the high energy state, the diffusion activation energy of grain boundaries was lower than that of the crystals. Thus, the solute atoms in the grain boundaries and surface transited more easily, and the diffusion rate was faster than that in the crystals internal. Because many metallography defects gathered at grain boundaries, lattice distortion happened. The atomic energy at grain boundary also increased, which was beneficial to the diffusion of the solute atoms and made them gather at the grain boundary. The position which was occupied by the solute atoms in the solid solution was not completely uniform, and the concentration of solute atoms in some regions was high, while it was low in other regions. The vacancy at the grain boundaries was also higher than that in grains, and the size was unequal. Large atoms occupied the large
space, and the small space was occupied by small atoms, which was the cause of the formation of grain boundary segregation. Thus, we can still observe the general shape of titanium hydride grains.
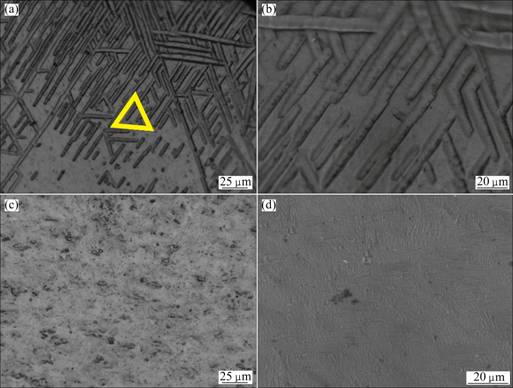
Fig. 4 Metallographs of samples before (a, b) and after (c, d) hydrogenation at 400 °C for 2 h
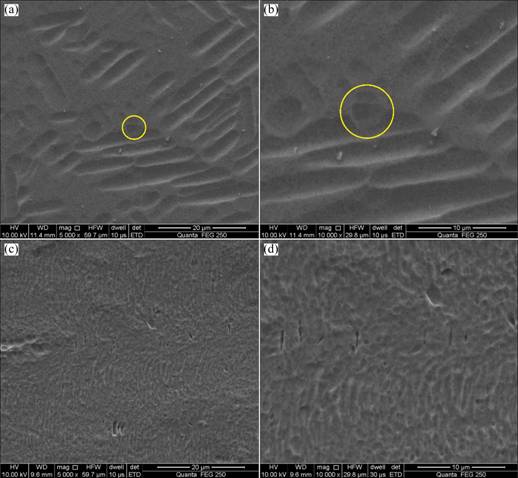
Fig. 5 SEM images of samples before (a, b) and after (c, d) hydrogenation at 400 °C for 2 h
4 Conclusions
1) The hydrogen content of titanium hydride could reach 2.52% after hydrogenation at 350 °C. At 500 °C, the hydrogen content of titanium hydride reached the highest value of 3.64%. Increasing the hydrogenation temperature was not conducive to obtain titanium hydride with high hydrogen content, while oxygen content increased.
2) The effect of hydrogenation time on hydrogen content of titanium hydride was not obvious. However, when the time was too long, the oxygen content would increase. The appropriate parameters of the hydrogenation process were heating at 400 °C and holding for 2 h. Under such condition, titanium hydride with hydrogen content of 3.63% and oxygen content of 0.18% was obtained.
3) The density of titanium hydride was lower than that of electrolyzed metallic titanium, and it decreased with increasing the hydrogen content. The micro hardness of titanium hydride was higher than that of electrolyzed metallic titanium, and it decreased with increasing the hydrogenation time.
4) Phase analysis showed that the main phases of titanium hydride were TiH1.924, TiH1.971 and TiH2. Because of the hydrogen atoms that went into the TiH2 with solid solution, the lattice parameters and interplanar crystal spacing increased.
5) The α-tissue of electrolyzed metallic titanium had three specific growth directions at 60° to each other with dense arrangement. After hydrogenation, these microstructures disappeared and the titanium hydride became homogeneous.
References
[1] ELIAS C N, MEYERS M A, VALIEV R Z, MONTEIROA S N. Ultrafine grained titanium for biomedical applications: An overview of performance [J]. Journal of Materials Research and Technology, 2013, 2(4): 340-350.
[2] RIPAMONTI U, RODEN L C, RENTON L F. Osteoinductive hydroxyapatite-coated titanium implants [J]. Biomaterials, 2012, 33(15): 3813-3823.
[3] BOLZONI L, RUIZ-NAVAS E M, GORDO E. Powder metallurgy CP-Ti performances: Hydride-dehydride vs sponge [J]. Materials and Design, 2014, 60(8): 226-232.
[4] LI Rui-di, SHI Yu-sheng, WANG Zhi-gang, WANG Li, LIU Jin-hui, JIANG Wei. Densification behavior of gas and water atomized 316L stainless steel powder during selective laser melting [J]. Applied Surface Science, 2010, 256(13): 4350-4356.
[5] LI Rui-di, LIU Jin-hui, SHI Yu-sheng, WANG Li, JIANG Wei. Balling behavior of stainless steel and nickel powder during selective laser melting process [J]. International Journal of Advanced Manufacturing Technology, 2012, 59(9-12): 1025-1035.
[6] ABKOWITZ S, ABKOWITZ S M, FISHER H. Homogeneous titanium tungsten alloys produced by powder metal technology: US, 8741077 [P]. 2014-6-3.
[7] KAWASAKI H, OHSHIMA T, ARAFUNE K , SUDA Y. Preparation of a titanium thin film using a sputtering deposition process with a powder material target [J]. Transactions of the Materials Research Society of Japan, 2012, 37(2): 147-150.
[8] BHOWMIK S, BENEDICTUS R, POULIS J A, BONIN H W, BUI V T. High-performance nanoadhesive bonding of titanium for aerospace and space applications [J]. International Journal of Adhesion and Adhesives, 2009, 29(3): 259-267.
[9] WANG Fang, CUI Wei-cheng, PAN Bin-bin, SHEN Yun-sheng, HUANG Xiao-ping. Normalised fatigue and fracture properties of candidate titanium alloys used in the pressure hull of deep manned submersibles [J]. Ships and Offshore Structures, 2014, 9(3): 297-310.
[10] OH J, ROH K, LEE B, SUH C, KIM W, KWON H, LIM J. Preparation of low oxygen content alloy powder from Ti binary alloy scrap by hydrogenation–dehydrogenation and deoxidation process [J]. Journal of Alloys and Compounds, 2014, 593: 61-66.
[11] SMALL D A, MACKAY G R, DUNLAP R A. Hydriding reactions in ball-milled titanium [J]. Journal of Alloys and Compounds, 1999, 284: 312-315.
[12] AZEVEDO C, AZEVEDO D, RODRIGUES F, BENEDUCE N. Ti-Al-V powder metallurgy (PM) via the hydrogenation dehydrogenation (HDH) process [J]. Journal of Alloys and Compounds, 2003, 353: 217-227.
[13] GU D, HAGEDORN Y, MEINERS W, MENG G, BATISTA R, WISSENBACH K, POPRAWE R. Densification behavior, microstructure evolution, and wear performance of selective laser melting processed commercially pure titanium [J]. Acta Materialia, 2012, 60(9): 3849-3860.
[14] PARTHASARATHY T A, PORTRE W J, BOONE S, JOHNC R, MATTIN P. Life prediction under tension of titanium alloys that develop an oxygenated brittle case during use [J]. Scripta Materialia, 2011, 65(5): 420-423.
[15] OH J M, KWON H, KIM W, LIM J W. Oxygen behavior during non-contact deoxidation of titanium powder using calcium vapor [J]. Thin Solid Films, 2014, 551: 98-101.
[16] OH J M, LEE B K, SUH C Y, CHO S W, LIM J W. Deoxidation of Ti powder and preparation of Ti ingot with low oxygen concentration [J]. Materials Transactions, 2012, 53(6): 1075-1077.
[17] WENG Q G, LI R D, YUAN T C, HE Y H. Investigation to impurity content and micromorphology of high purity titanium powder prepared by molten salt electrolysis [J]. Materials Research Innovations, 2013, 17(6): 396-402.
[18] WENG Qi-gang, LI Rui-di, YUAN Tie-chui, LI Jian, HE Yue-hui. Valence states, impurities and electrocrystallization behaviors during molten salt electrorefining for preparation of high-purity titanium powder from sponge titanium [J]. Transactions of Nonferrous Metals Society of China, 2014, 24(2): 553-560.
[19] OH J M, LEE B K, SUH C Y, CHO S W, LIM J W. Preparation method of Ti powder with oxygen concentration of <1000 ppm using Ca [J]. Powder Metallurgy, 2012, 55(5): 402-404.
[20] GENG F, NIINOMI M, NAKAI M. Observation of yielding and strain hardening in a titanium alloy having high oxygen content [J]. Materials Science and Engineering A, 2011, 528(16): 5435-5445.
[21] YU Cun-ye. Effects of “iron” on the service of chemical equipment made of titanium [J]. Corrosion and Protection, 2005, 26(9): 407-410.
[22] YAN M, LUO S D, SCHAFFER G B, QIAN M. Impurity (Fe, Cl, and P)-induced grain boundary and secondary phases in commercially pure titanium (CP-Ti) [J]. Metallurgical and Materials Transactions A, 2013, 44(8): 3961-3969.
[23] HU Ang-gang, CAO Xiao-hua, LONG Xing-gui. Physical and chemical properties of titanium-hydrogen system [J]. Materials Review, 2006, 20(10): 128-134.
[24] XU J, CHEUNG H, SHI S. Mechanical properties of titanium hydride [J]. Journal of Alloys and Compounds, 2007, 436(1): 82-85.
熔盐电解金属钛的氢化反应
翁启钢1,李瑞迪1,袁铁锤1,史玉升2,邱子力1,蒋明祥1,贺跃辉1
1. 中南大学 粉末冶金国家重点实验室,长沙 410083;
2. 华中科技大学 材料成形与模具技术国家重点实验室,武汉 430074
摘 要:研究电解钛的氢化反应,电解钛的氢化反应是以电解钛为原料通过氢化脱氢制取高纯钛粉的第一步。在不同的氢化温度和时间下,通过电解钛与氢气反应制备氢化钛,研究不同氢化条件下,氢化钛内氢和氧含量、密度、显微硬度和相成分在氢化前后的变化规律。结果表明:电解钛经氢化后氢化物主要相为TiH1.924、TiH1.971和TiH2。升高温度不能增加氢含量却使氧含量增加。氢化时间对氢含量的影响不明显。电解钛氢化的较佳工艺参数为400 °C下保温2 h,可获得氢含量为3.63%、氧含量为0.18%(质量分数)的氢化钛。同时,研究电解钛及氢化钛的显微组织、织构及晶体学取向。
关键词:钛粉;氢化反应;熔盐电解;氢含量
(Edited by Mu-lan QIN)
Foundation item: Projects (51474245, 51571214) supported by the National Natural Science Foundation of China; Projects (2015GK3004, 2015JC3006) supported by the Science and Technology Project of Hunan Province, China; Project (P2014-07) supported by the Open Fund of State Key Laboratory of Materials Processing and Die & Mould Technology, China
Corresponding author: Tie-chui YUAN; Tel: +86-731-88877322; E-mail: tiechuiyuan@csu.edu.cn
DOI: 10.1016/S1003-6326(16)64219-X
Abstract: The hydrogenation reaction of electrolyzed titanium, as the first step during hydrogenation-dehydrogenation for the preparation of titanium powder, was studied. The titanium hydride was prepared through the reaction between electrolyzed titanium and hydrogen at different hydrogenation temperatures and different time. The evolutions of hydrogen and oxygen contents, density, hardness and phase composition before and after hydrogenation were characterized under different hydrogenation conditions. The results show that the main phases of titanium hydride were TiH1.924, TiH1.971 and TiH2. Increasing the hydrogenation temperature could not enhance the hydrogen content but increase the oxygen content. The effect of the hydrogenation time on the hydrogen content was not obvious. The optimal parameters of the hydrogenation process were obtained: heating at 400 °C and holding for 2 h, by which the hydrogen content of 3.63% and oxygen content of 0.18% (mass fraction) can be obtained. In addition, the microstructure, orientations and tissues of electrolyzed titanium and titanium hydride were detected.


