
Bioleaching of Zn(Ⅱ) and Pb(Ⅱ) from Nigerian sphalerite and galena ores by mixed culture of acidophilic bacteria
Alafara A. BABA1, Folahan A. ADEKOLA1, Rasaq F. ATATA2,
Risikat N. AHMED3, Sandeep PANDA4
1. Department of Chemistry, University of Ilorin, P.M.B. 1515, Ilorin 240003, Nigeria;
2. Department of Biological Sciences, Fountain University, P.M.B 4491, Osogbo 230001, Nigeria;
3. Department of Microbiology, University of Ilorin, P.M.B. 1515, Ilorin 240003, Nigeria;
4. Bioresources Engineering Department, Institute of Minerals and Materials Technology,Bhubaneswar 751013, India
Received 8 November 2010; accepted 25 August 2011
Abstract:
Zn(Ⅱ) and Pb(Ⅱ) from Nigerian sphalerite and galena ores were bioleached by a mixed culture of acidophilic bacteria. The influences of pH and ferric ion on the bioleaching rates of sphalerite and galena were examined. The result shows that pH 2.1 and 2.7 are favourable for the leaching of Zn(Ⅱ) and Pb(Ⅱ) from sphalerite and galena, respectively. It was observed that the use of agarose-simulated media caused cells to excrete exopolymers containing ferric ions which enhanced oxidation. The oxidation equilibrium for sphalerite and galena took 3 and 4 d, respectively. About 38.3% sphalerite and 34.2% galena were leached within 1 d and approximately 92.0% Zn(Ⅱ) and 89.0% Pb(Ⅱ) were recovered in 5 d, respectively. The unleached residual products were examined by X-ray diffraction for sphalerite, revealing the presence of elemental sulphur(S), zinc sulphate (ZnSO4) and few traces of calcium aluminate (Ca3Al2O6). The XRD pattern also indicates the presence of elemental sulphur (S), lead sulphate (PbSO4) and few traces of itoite [Pb(S,Ge)(O,OH)4] and cobalt lead silicate [Pb8Co(Si2O7)3] in the unleached galena ore.
Key words:
sphalerite; galena; zinc (Ⅱ); lead (Ⅱ); oxidation; acidophilic bacteria;
1 Introduction
Sulphide ores including sphalerite and galena exhibit similarities and complexities in their mineralogical associations and properties, which often pose challenges during their hydrometallurgical processing. As a result, increasing efforts have been recently focused on the application of biohydrometallurgical processing routes for recovering their constituent metals. Nevertheless, the complexities of these sulphide ores and their general low solubility also place a limitation during biohydrometallurgical processing [1].
The use of micro-organisms in metal solubilization processes has attracted much attention from geomicrobiologists and metallurgist in recent years. The leaching of metals using micro-organisms is very attractive because of its low cost and relatively few pollution problems compared with conventional hydrometallurgical process. Metals are commonly associated with various forms of inorganic sulphides in nature. It has been well documented that these metals are constantly solubilized by microbiological action in nature [2].
Consequently, Acidithiobacillus ferrooxidans, an acidophilic chemolithotroph can derive energy by oxidizing ferrous ions or from reduced forms of sulphur compounds. The oxidation of sulphur compounds results in the solubilization of heavy metals, by lowering the pH of the environment. Acidithiobacillus ferrooxidans is known to develop extraordinary tolerance to metals which are often toxic to other micro-organisms. Numerous researchers [3-7] have documented the application of Acidithiobacillus ferrooxidans in the leaching of heavy metals including copper, zinc, lead, uranium, silver and gold.
There is currently great interest in the bioleaching of sulphide minerals, especially for the processing of copper and zinc, and little attention has been paid to the bacteria oxidation of galena, largely due to the fact that in a sulphate system, galena is oxidized to insoluble lead sulphate. The formation of lead sulphate prohibits the recovery of lead from bacterial and ferric sulphate leaching via traditional solvent extraction/electrowinning routes, and also creates an environmental hazard due to the increasing solubility of lead sulphate over galena [5, 8].
As applicable to other sulphidic minerals, BOON et al[4] have postulated that the microbial leaching of galena or sphalerite often proceeds through an indirect mechanism according to the following relations:
MeS+2Fe3+→Me2++S+2Fe2+ (1)
where MeS signifies sulphide minerals in the form of Me2+S2- (e.g., PbS or ZnS)
2Fe2++0.5O2+2H+![]() 2Fe3++H2O (2)
2Fe3++H2O (2)
S+1.5O2+H2O![]() SO42-+2H+ (3)
SO42-+2H+ (3)
Using these routes, bacteria are taught to accelerate the reaction rate by oxidizing the elemental sulphur product layer. Thus, possible diffusion resistance is removed and the concentration of sulphuric acid is also increased.
In general, it is important to note that there is practically little or no work done on the application of microbial approach to the leaching of Nigerian sphalerite and galena ores. An attempt made by BABA et al[9] to dissolve the ore via microbial action of Acidithiobacillus ferrooxidans was not successful, and it was attributed probably to very low sulphur content of the mineral. Therefore, in spite of the importance of the bacterial oxidation of sphalerite and galena, this study is aimed at understanding the leaching behaviour of Zn(Ⅱ) and Pb(Ⅱ) oxidation from a Nigerian sphalerite and galena ores by a mixed culture of acidophilic bacteria predominantly Acidithiobacillus ferrooxidans in a agarose-simulated medium.
2 Experimental
2.1 Ore samples/Instrumentation
Two types of sulphidic minerals sphalerite and galena ores were investigated. The two ores were both of Nigerian origin and they were collected from the Nigerian Federal Ministry of Solid Minerals, Kaduna and Department of Geology and Mineral Sciences, University of Ilorin, Ilorin-Nigeria. Both ores were extracted from Abakaliki Mining Fields of South Eastern Nigeria.
The elemental analysis of the sphalerite and galena samples was carried out using X-ray fluorescence (XRF) technique (Philip’s model 120454/3), while the mineralogical purity was examined with the aid of Philip’s automatic powder (XRD) diffractometer system (PW1800), contained within a single cabinet. The cabinet housed a high speed, a high precision goniometer, a high efficiency generator (X-ray) and an automatic sampler.
2.2 Microbial leaching of sphalerite and galena
2.2.1 Micro-organisms and their growth on solid media
Samples obtained from Rust scrap at Motor dump site in Ipata-Oloje, Ilorin West Local Government Area of Kwara State, Nigeria, were cultured in 9K medium containing FeSO4·7H2O as energy source. The composition of 9K medium was 3.0 g/L (NH4)2SO4, 0.5 g/L MgSO4·7H2O, 0.01 g/L Ca(NO3)2, 5 mol/L H2SO4, 0.5 g/L K2HPO4 and 0.1 g/L KCl, 100 mL distilled water [10].
The pH value of the medium was adjusted to 2.0 by adding dilute H2SO4. The media was sterilized by autoclaving at 15 lbs for 10 min before being inoculated [11]. 10 g ore was added to 100 mL medium. The mixture was then shaked in AMPS Gallenkamp Orbital Shaker for 5 d at 100 r/min for effective aeration of the organisms. The preparation of Basal salt solid media was carried out according to the procedure adopted by YATES and HOLMES [12].
2.2.2 Microbial leaching study
The basal salt culture medium of 1 mL was plated on plates containing the solid media and incubated at 35 °C. This procedure was carried out on daily basis for 9 d to determine the growth rate of bacteria in the medium and also to monitor the leaching rate of the sphalerite ore by the mixed culture. Colonies developed on the plate in 1-9 d were counted and recorded[2, 13-14]. Non-inoculated blanks were performed in all cases as negative control.
The influence of pH was examined for both sphalerite and galena microbial leach liquors when the highest growth was observed.
At the end of ore leaching process, the medium containing bacteria, agarose and mineral was sterilized in an autoclave to make the medium safe for further chemical analyses. The sterilized culture medium was kept for further chemical investigations. The sterilized culture medium was filtered using millipore filter paper with size of 45 μm. The filtrate from this medium was used in the spectrophotometric analysis of zinc and iron from the sphalerite leachate liquor by AQUAMATE Thermo-Electron Corporation UV/visible spectro- photometer, together with EPSON LQ 2070 recorder using 4-(pyridyl-2-azo)resorcinol, PAR method [15] for zinc and o-phenanthroline method for iron [16].
The same procedure was repeated for galena ore. The lead and iron contents in the galena leached liquor were also determined spectrophotometrically using PAR method, respectively [16-17].
In each case, residual products were then air-dried and oven-dried at 60 °C for 24 h and then subjected to X-ray diffraction analysis (XRD, Philips Scientific) with Cu Kα target (40 kV, 55 mA) for the identification of the mineral phases before and after microbial attack.
3 Results and discussion
3.1 Characterization of sphalerite and galena ores
3.1.1 Elemental analysis by XRF
The X-ray fluorescence (XRF) analysis on the composition of sphalerite ore used for this work has earlier been reported [18] with Zn (44.7%), S (32.3%), Fe (7.39%), Sn (4.21%) and Ag (1.62%) constituting the major elements in the ore. The major elements in galena ore include Pb (58.7%), S (13.3%), Sn (2.7%), Fe (0.5%) and Zn (0.16%) [19]. The results of the elemental analysis by XRF technique show that the sphalerite and galena ores exist mainly as ZnS and PbS, respectively.
3.1.2 Phase studies by XRD
The X-ray diffraction analysis of the sphalerite and galena gives better understanding in terms of the mineral phases present in the ores. The results of our recent findings show that the X-ray diffractogram of the sphalerite ore [20] reveals the presence of sphalerite (ZnS) as the most intense peak. Other compounds identified include α-quartz (α-SiO2), ilmenite (FeTiO3), cassiterite (SnO2), pyrite (FeS2), hausmanite (Mn3O4) and baddeleyite (ZrO2).
The X-ray diffraction data of the galena ore[19] identifies galena (PbS) as the most intense peak. Other compounds identified are cassiterite (SnO2), α-quartz (α-SiO2), pyrite (FeS2), sphalerite (ZnS) and manganese oxide (MnO2).
3.2 Microbial investigation
3.2.1 Colony growth rate of acidophilic bacterium in sphalerite and galena bioleaching
The results of colony growth rate of acidophilic bacterium in sphalerite and galena bioleaching are shown in Fig. 1.
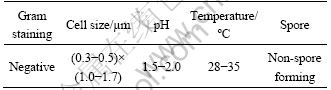
The photomicrographs of the isolated mixed culture as observed on a petri plate and under field emission scanning electron microscope (FESEM, Zeiss Supra 55) are shown in Fig. 2. The morphological characterization of the isolated bacteria is summarized in Table 1.
It is observed from Fig. 1 that the highest rate of the bacterial cells growth occurs at 2 d, which exhibits as high as 475 and 328 cfu/mL for sphalerite and galena, respectively, then a decline is observed from 3 d. This observation indicates the probability of nutrient depletion as a result of competition for the survival of organisms for growth factors, avoiding positive oxidation.
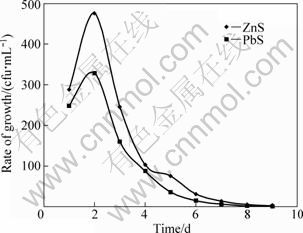
Fig. 1 Growth rate of acidophilic bacteria monitored on daily basis for sphalerite and galena bioleaching (Experimental conditions: concentration of ore=10 g/L; mass of nutrient agar=7 g; volume of cultured media=250 mL; duration of aeration=3-5 d; temperature of incubator=35 °C; sterilizing temperature=121C and 160 °C for steam and heat sterilization, respectively; agitation period=430 and 320 min for sphalerite and galena, respectively)

Fig. 2 Photo of colonial appearance of acidophilic bacteria on culture plate (a) and SEM image of rod shaped bacteria (b)
Table 1 Morphological characterization of isolated bacteria
From this study and what has been reported in Ref. [21], bioleaching was analyzed after 48 h incubation. This time is required by the bacteria for the full growth and adaptation in the solid substrate system (ore), which can avoid the initial lag-phase [2, 22]. Therefore, the leaching efficiency by the organisms is dependent on the growth rate of the organisms. Consequently, the reduced growth of organism with increase in time decreases gradually, while the organism potential increases as a result of increasing oxidation.
3.2.2 Effects of pH on sphalerite and galena bioleaching
The effect of pH on the bioleaching rate of sphalerite and galena was investigated over the pH range of 1.0-6.0. The pH of the mixture is adjusted by adding 2 mol H2SO4 on daily basis. The results of these findings are shown in Fig. 3 for sphalerite and galena, respectively.
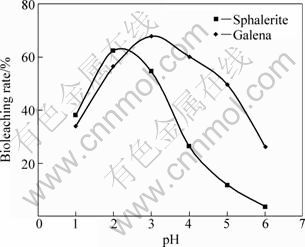
Fig. 3 Effect of pH on bioleaching rate
The results from Fig. 3 show that the bioleaching rate of sphalerite initially increases from pH 1 to pH 2, after which it decreases with increasing pH. This is attributed to the suppression of bacterial activity [23]. Therefore, with the increase in pH, the bioleaching rate sharply declines. The optimum pH value of 2.1 is taken afterwards for process of parameter optimization studies.
However, the results from Fig. 3 reveal a general increase in pH which favours the galena oxidation at the initial stage of bioleaching, after which the pH decreases slowly. This trend has been recently observed by OLUBAMBI et al [1] on the effects of ore mineralogy on the microbial leaching of low grade complex sulphide ores. Hence, the initial rise in pH could be attributed to the dissolution of the soluble and high acid consuming galena, while the progressive decrease in pH could be a result of iron hydrolysis with the precipitation of ferric compounds and the oxidation of sulphur. In this study, the optimum pH for galena bioleaching is found to be 2.7.
3.2.3 Effects of ferrous ions on sphalerite and galena bioleaching
The addition of ferrous ions into the leaching system enhances the rate of sphalerite oxidation, giving a recovery over 80% for zinc(Ⅱ) in 5 d. This result is more favourable than that obtained after leaching for 8 d where zinc sulphide was used as the sole energy source [21]. When 10 g/L ferrous ions were added to 10 g/L sphalerite medium, complete oxidation of ferrous ions by the micro-organism takes place within 72 h, followed by the sphalerite oxidation (Fig. 4).
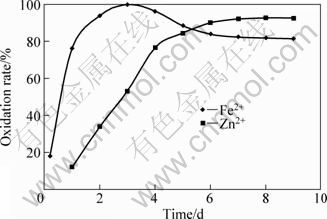
Fig. 4 Oxidation of ferrous and zinc ions in sphalerite leaching medium (Experimental conditions: temperature=35 °C; pH=2.1; concentration of FeSO4=10 g/L; concentration of sphalerite=10 g/L; particle diameter = 63-112 μm; bacterial growth = 475 cfu/mL.)
The results illustrated in Fig. 4 indicate that the micro-organism prefers ferrous ions to Zn ions as an energy source. This observation is in conformity with the earlier published works [21, 23-24] and very recently ones by PLUMB et al[25] on the effect of pH on rates of iron and sulphur oxidation by bioleaching organisms. However, as proposed by BANG et al [2], complete oxidation of ferrous ions by the micro-organism takes place in 24 h.
Conversely, the addition of ferrous ions into the leaching medium improves the rate of galena oxidation, giving a recovery over 80% lead (Ⅱ) in 6 d. This result also exhibits an improvement compared with 8 d post-leaching where lead sulphide was used as the sole energy source and a recovery of about 67.7% was attained [2] when 10 g/L ferrous ions were added to 10 g/L galena medium, complete oxidation of ferrous ions by the micro-organism took place after 96 h (4 d), followed by galena oxidation, as shown in Fig. 5.
The results of this investigation show that ferrous ion enhances bioleaching of galena. This observation has equally been observed with sphalerite. The oxidation equilibrium for galena bioleaching took 4 d and that of sphalerite took 3 d. In each case, these observations are in agreement with the works by PLUMB et al [25], RODRIGUEZ et al [26] and SANTHIYA et al [27].
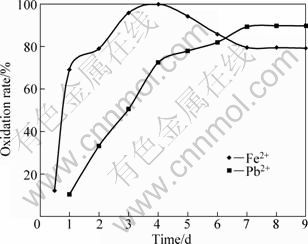
Fig. 5 Oxidation of ferrous and lead ions in galena leaching medium (Experimental conditions: temperature=35 °C; pH=2.7; concentration of FeSO4=10 g/L; concentration of sphalerite=10 g/L; particle diameter=63-112 μm; bacterial growth=328 cfu/mL)
3.2.4 Influence of sphalerite and galena by ferric ion in bacterial medium
As seen from Figs. 4 and 5, some of the ferric ions in the sphalerite and galena media are respectively reduced back as the leaching of sphalerite continues, leading to 82% and 80% oxidized ferrous ions. The involvement of ferric ions in sphalerite and galena leaching is respectively summarized in Figs. 6 and 7, where ferrous ions were introduced to the micro-organism as a sole energy source [2].
The ferrous ions are completely oxidized within 72 h (Fig. 5) for sphalerite and 92 h for galena, respectively (Fig. 5). The culture was then treated at 60 °C in a water bath for 30 min to ensure the complete death of bacteria. To confirm the death of all viable cells, heat-treated cells were used as inoculants of the new medium in which no cell growth was observed. The leaching flask was then supplemented with 10 g/L sphalerite (marked by an arrow is Fig. 6) and returned to the shaker. The same procedure was done to galena (Fig. 7).
From Figs. 6 and 7, ferric ions immediately participate in sphalerite and galena oxidation, respectively. Consequently, 38.28% sphalerite and 34.16% galena are leached within 1 d and approximately 92.0% total zinc(Ⅱ) and 89.0% total lead(Ⅱ) are recovered in 5 d, respectively.
The results of the experiments suggest that H+ ion concentration initially decreases due to the acid consumption from the proton attack of sphalerite and galena, and increases again because of the elemental sulphur oxidation by sulphur oxidizing micro-organisms. This observation could be attributed to the fact that kinetics and efficiency of bioleaching are directly related to the production of Fe3+ and H+ ions in solution [23, 28-29].
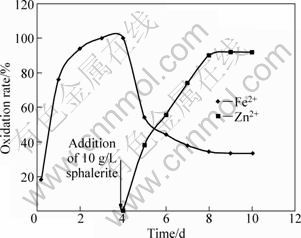
Fig. 6 Influence of sphalerite by ferric ion in bacterial medium when organisms were killed after 4 d at which 10 g/L sphalerite was added (Experimental conditions: temperature=35 °C; concentration of FeSO4=10 g/L, particle diameter=63-112 μm; bacterial growth=475 cfu/mL; pH=2.1)
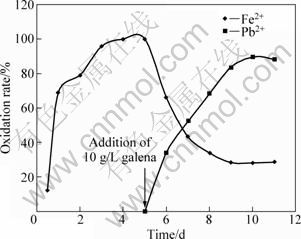
Fig. 7 Influence of galena leaching by ferric ions in bacterial medium when bacteria were killed after 5 d when 10 g/L galena was added (Experimental conditions: temperature=35 °C, pH=2.7; concentration of FeSO4=10 g/L; particle diameter= 63-112 μm; bacterial growth=328 cfu/mL)
3.2.5 Characterization of sphalerite and galena bioleaching residual products
The bioleaching experiments were carried out on sphalerite and galena samples with particle diameter from 63 to 112 μm, and the X-ray diffraction analysis was performed on solid sample which gives the highest Zn(Ⅱ) and Pb(Ⅱ) oxidation, as shown in Figs. 7 and 8, respectively, in order to examine the mineral phases that were left unattacked by the micro-organism. Consequently, the results of these investigations are summarized in Figs. 8 and 9, respectively.
Both the elemental sulphur (S) and zinc sulphate (ZnSO4) are the major insoluble products at the end of sphalerite bioleaching. Few traces of calcium aluminate (Ca3Al2O6) are also detected by XRD. However, the XRD pattern in Fig. 9 indicates the presence of elemental sulphur (S), lead sulphate, (PbSO4) and few traces of itoite [Pb(S,Ge)(O,OH)4], cobalt lead silicate and [Pb8Co(Si2O7)] are the unleached products which are not attacked by the bacteria in the galena ore.
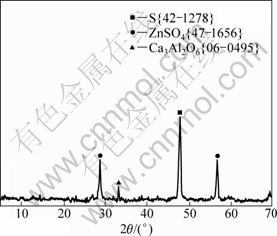
Fig. 8 XRD pattern of sphalerite bioleaching residual product
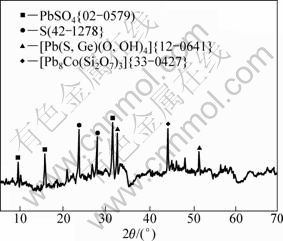
Fig. 9 XRD pattern of galena bioleaching residual product
4 Conclusions
1) The sphalerite and galena leaching is found to be effective at solution pH of 2.1 and 2.7, respectively.
2) The use of the selective medium allows for the excretion of exopolymers by the bacteria and this medium contains ferric ions which enhance oxidation and attack of the ores, leading to 83.4% Zn(Ⅱ) and 80% Pb(Ⅱ) recovery efficiencies from sphalerite and galena ores, respectively.
3) Ferric ions immediately participate in sphalerite and galena oxidation, showing a drastic decrease in the ferric ion concentration with addition of 10 g/L sphalerite and galena, respectively, to the bacterial medium. About 38.28% sphalerite and 34.16% galena are leached within 1 d and approximately 92.0% Zn(Ⅱ) and 89.0% Pb(Ⅱ) are recovered in 5 d, respectively.
4) The unleached products for sphalerite ore obtained at the end of bioleaching experiments contain elemental sulphur, zinc sulphate and few traces of calcium aluminate by XRD. While the X-ray diffractogram indicates the presence of elemental sulphur, lead sulphate, and few traces of itoite and cobalt lead silicate in the residual galena.
5) The results of the bioleaching experiments exhibit higher efficiency for sphalerite oxidation compared with galena oxidation by the mixed culture of acidophilic bacteria.
Acknowledgements
A. A. BABA wishes to thank University of Ilorin, Ilorin-Nigeria for the 2005/2006 Staff Development Award for Ph.D research and for permission to honor CSIR-TWAS Postdoctoral Fellowship Award; Prof. B. K. MISHRA, the director of Institute of Minerals and Materials Technology, Bhubaneswar-751013 for the acceptance as CSIR-TWAS Postdoctoral Research Fellow and the Management, Academy of Sciences for the Developing World, Trieste, Italy for the Award of 2010 CSIR-TWAS Fellowship for Postdoctoral Research (May 2011-January 2012). Finally, to Mr. J.O. FALAJA of N.N.P.C. Abuja for financial assistance on the XRD analysis.
References
[1] OLUBAMBI P A, NDLOVU S, POTGIETER J H, BORODE J O. Effects of ore mineralogy on the microbial leaching of low grade complex sulphide ores [J]. Hydrometallurgy, 2007, 86(1-2): 96-104.
[2] BANG S S, DESHPANDE S S, HAN K N. The oxidation of galena using Thiobacillus ferrooxidans [J]. Hydometallurgy, 1995, 37(2): 181-192.
[3] LUNDGREN D G, SILVER M. Ore leaching by bacteria [J]. Annual Rev. Microbiol, 1980, 34: 263-283.
[4] BOON M, SNIJDER M, HANSFORD G S, HEIJNEN J J. The oxidation kinetics of zinc sulphide with T. ferroxidans [J]. Hydrometallurgy, 1998, 48 (2): 171-186.
[5] LIAO M X, DENG T L. Zinc and lead extraction from complex raw sulphides by sequential bioleaching and acidic brine leach [J]. Miner Eng, 2004, 17(2): 17-22.
[6] da-SILVA G. Kinetics and mechanism of the bacterial and ferric sulphate oxidation of galena [J]. Hydrometallurgy, 2004, 75: 99-110.
[7] GIAVENO A, LAVALLE L, CHIACCHIARINI P, DONTI E. Bioleaching of zinc from low-grade complex sulphide ores in an airlift by isolate ferrooxidans [J]. Hydrometallurgy, 2007, 89(1-2), 117-126.
[8] FOWLER T A, CRUNDWELL F K. Leaching of zinc sulphide by Thiobacillus ferrooxidans: Bacterial oxidation of the sulphur product layer increases the rate of zinc sulphide dissolution at high concentrations of ferrous ions [J]. Appl Environ Microb, 1999, 65(12): 5285-5292.
[9] BABA A A, ADEKOLA F A, LAWAL A J. Investigation of chemical and microbial leaching of iron ore in sulphuric acid [J]. J Appl Sci Environ Mgt, 2007, 11(1): 39-44.
[10] LENNOX J E, BLAHA T. Leaching of copper ore by Thiobacillus ferrooxidans [J]. The American Biology Teacher, 1991, 53(6): 361-368.
[11] YU Run-lan, TAN Jian-xi, GU Guo-hua, HU Yue-hua, QIU Guan-zhou. Mechanism of bioleaching chalcopyrite by Acidithiobacillus ferrooxidans in agar-simulated extracellular polymeric substances media [J]. Journal of Central South University of Technology, 2010, 17(1): 56-61.
[12] YATES J R, HOLMES D S. Two families of repeated DNA sequences in Thiobacillus ferrooxidans [J]. Journal of Bacteriology, 1987, 169(5): 1861-1870.
[13] REZZA I, SALINAS E, CALVENTE V, BENUZZI D, Sanz de TOSETTI M I. Extraction of lithium from spodumene by bioleaching [J]. Letters in Appl Microbiology, 1997, 25(3): 172-176.
[14] PINA P S, LEAO V A, SILVA C A, DAMAN D, FRENAY J. The effect of ferrous and ferric iron on sphalerite bioleaching with Acidithiobacillus sp [J]. Min Engr, 2005, 18(5): 549-551.
[15] GOMEZ E, ESTELA J M, CERDA V, BLANCO M, Simultaneous Spectrophotometric determination of metal ions with 4-(Pyridyl-2-azo) resorcinol (PAR) [J]. Frensenius J Anal Chem, 1992, 342: 318-321.
[16] MALATI M A. Experimental inorganic/physical chemistry [M]. Chrichester, England: Horwood Publishing, 1999: 186-187.
[17] YADAV A A, KHOPKAR S M. Liquid-liquid extraction of lead(Ⅱ) with tributylphosphate [J]. Talanta, 1971, 18(8): 833-837.
[18] BABA A A, ADEKOLA F A, MESUBI M A, BALE R B. The characterization and lixiviation of sphalerite mineral in some acidic media [J]. J Chem Soc Nigeria, 2003, 28(1): 40-44.
[19] BABA A A, ADEKOLA F A. Comparative analysis of the dissolution kinetics of galena in binary solutions of HCl/FeCl3 and HCl/H2O2 [J]. International Journal of Minerals, Metallurgy and Materials, 2011, 18(1): 9-17.
[20] BABA A A, ADEKOLA F A. Hydrometallurgical processing of a Nigerian sphalerite in hydrochloric acid: Characterization and dissolution kinetics [J]. Hydrometallurgy, 2010, 101(1-2): 69-75.
[21] HOSSAIN S M, DAS M, BEGUM S M, ANANTHARANMAN N. Bioleaching of zinc sulphide (ZnS) ore using Thiobacillus ferroxidans [J]. Journal of the Institution of Engineers, 2004, 85: 7-11.
[22] HOSSAIN S M, DAS M, IBRAHMIN S H. Scale-up and optimization studies on lignin bio-degration of rice-straw using Phanerochaete Chrysosporium [J]. Ind J Chem Techn, 2002, 9(3): 227-234.
[23] BALLESTER A, RODRIGUEZ Y, BLASZQUEZ M L, GONZALEZ F, MUNOZ J A. New information on the sphalerite bioleaching mechanism at low and high temperature [J]. Hydrometallurgy, 2003, 71(1): 57-66.
[24] RAWLINGS D E, DEW D, DU-PLESSIS C. Biomineralization of metal-containing ores and concentrates [J]. Trends in Biotechnol, 2003, 21(1): 38-44.
[25] PLUMB J J, MUDDLE R, FRANZMANN P D. Effect of pH on rates of iron and sulphur oxidation by bioleaching organisms [J]. Min Engr, 2008, 21(1): 76-82.
[26] RODRIGUEZ Y, BALLESTER A, BLAZQUEZ M L, GONAZALEZ F, MUNOZ J A. New information on the pyrite bioleaching mechanism at low and high temperature [J]. Hydrometallurgy, 2003, 71: 37-46.
[27] SANTHIYA D, SUBRAMANIAN S, NATARAJAN K A. Surface chemical studies on galena and sphalerite in the presence of Thiobacillus ferrooxidans with reference to mineral beneficiation [J]. Min Engr, 2000, 13: 747-763.
[28] SAND W, GEHRKE T, JOZSA P G, SCHIPPERS A. Direct versus indirect bioleaching [C]//Proceedings of the International Biohydrometallurgy Symposium, IBS’99 EI Escorial. Amsterdam: Elsevier, 1999, A: 27-49.
[29] SCHIPPERS A, SAND W. Bacterial leaching of metal sulphides proceeds by two indirect mechanisms via Thiosulphate or via polysulphides and sulphur [J]. Appl and Environ Microbiology, 1999, 65(1): 319-321.
利用嗜酸混合菌从尼日利亚闪锌矿和
方铅矿中生物浸出Zn(Ⅱ)和Pb(Ⅱ)
Alafara A. BABA1, Folahan A. ADEKOLA1, Rasaq F. ATATA2,
Risikat N. AHMED3, Sandeep PANDA4
1. Department of Chemistry, University of Ilorin, P.M.B. 1515, Ilorin 240003, Nigeria;
2. Department of Biological Sciences, Fountain University, P.M.B 4491, Osogbo 230001, Nigeria;
3. Department of Microbiology, University of Ilorin, P.M.B. 1515, Ilorin 240003, Nigeria;
4. Bioresources Engineering Department, Institute of Minerals and Materials Technology,
Bhubaneswar 751013, India
摘 要:利用嗜酸混合菌在尼日利亚闪锌矿和方铅矿中生物浸出Zn(Ⅱ)和Pb(Ⅱ),研究pH值和铁离子对闪锌矿和方铅矿浸出率的影响。结果表明:闪锌矿和方铅矿中Zn(Ⅱ)和Pb(Ⅱ)的氧化物在pH值分别为2.1和2.7时浸出效果最好。用模拟的琼脂糖培养基使细胞分泌出含铁离子的外聚合物,以此加强氧化。闪锌矿和方铅矿达到氧化平衡分别需要3和4 d。闪锌矿和方铅矿在1 d内的浸出率分别为38.3%和34.2%,5 d内Zn(Ⅱ)和Pb(Ⅱ)分别还原92.0%和89.0%。使用XRD研究两种矿物的未浸出的剩余产物,闪锌矿的组成为S、ZnSO4和少量Ca3Al2O6,方铅矿的组成为S、PbSO4和少量的Pb(S,Ge)(O,OH)4 和Pb8Co(Si2O7)3。
关键词:闪锌矿;方铅矿;Zn(Ⅱ);Pb(Ⅱ);氧化;嗜酸菌
(Edited by FANG Jing-hua)
Corresponding author: Alafara A. BABA; Tel: +23-48035010302; E-mail: alafara@unilorin.edu.ng
DOI: 10.1016/S1003-6326(11)61047-9
Abstract: Zn(Ⅱ) and Pb(Ⅱ) from Nigerian sphalerite and galena ores were bioleached by a mixed culture of acidophilic bacteria. The influences of pH and ferric ion on the bioleaching rates of sphalerite and galena were examined. The result shows that pH 2.1 and 2.7 are favourable for the leaching of Zn(Ⅱ) and Pb(Ⅱ) from sphalerite and galena, respectively. It was observed that the use of agarose-simulated media caused cells to excrete exopolymers containing ferric ions which enhanced oxidation. The oxidation equilibrium for sphalerite and galena took 3 and 4 d, respectively. About 38.3% sphalerite and 34.2% galena were leached within 1 d and approximately 92.0% Zn(Ⅱ) and 89.0% Pb(Ⅱ) were recovered in 5 d, respectively. The unleached residual products were examined by X-ray diffraction for sphalerite, revealing the presence of elemental sulphur(S), zinc sulphate (ZnSO4) and few traces of calcium aluminate (Ca3Al2O6). The XRD pattern also indicates the presence of elemental sulphur (S), lead sulphate (PbSO4) and few traces of itoite [Pb(S,Ge)(O,OH)4] and cobalt lead silicate [Pb8Co(Si2O7)3] in the unleached galena ore.


