
Fabrication of zirconia separator for nickel-hydrogen battery
L? Fang(吕 芳), LI Hua-ling(李华玲), JIANG Li-jun(蒋利军),
WANG Shu-mao(王树茂), LIU Xiao-peng(刘晓鹏), LI Zhi-nian(李志念), LI Guo-bin(李国斌)
Energy Materials and Technology Research Institute,General Research Institute for Nonferrous Metals, Beijing 100088, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Zirconia separator is one of the key materials of nickel-hydrogen battery, thereby zirconia separators are prepared by precursor process in which cellulose textiles immersed with zirconium salts are oxidized, infrared spectra show that viscose textile is an excellent precursor for preparing zirconia separator. The dominant factors in immersion are studied, it is revealed that the solution concentration and the temperature are the most important factors with regard to the area density of zirconia separator. The main reactions of immersed textiles during heat treatment are investigated by TG-DSC. The prepared zirconia separators are analyzed by SEM, XRD and infrared spectroscopy, which lead to kown that the separators maintain the same morphology of precursor textiles and contain little organic components, the main phase of the separators is tetragonal zirconia, the rate and the amount of alkaline absorption are about 5 cm/min and 220% respectively.
Key words:
zirconia; nickel-hydrogen battery separator; precursor process;
1 Introduction
Nickel-hydrogen (Ni-H2) batteries were developed initially by COMASAT Laboratory, US Air Force and Hughes Aircraft Company for use in low earth obit(LEO) craft and geosynchronous obits(GEO)[1]. They have higher gravimetric energy density and fewer inherent failure mechanisms than nickel-cadmium (Ni-Cd) batteries. Separator is one of the key materials of Ni-H2 batteries. In former designs, non-woven asbestos was used as the separator material. Currently asbestos has been replaced by zirconia separator which reduces the electrolyte redistribution and improves the operational life of Ni-H2 batteries. Zirconia separator is the best separator material used for Ni-H2 batteries. They are better at resistance to attack by electrolytes, morphology, thickness, bubble pressure, wicking rate, electrolyte retention and compressive, tensile strength than other kinds of separators.
In the US, ZIRCAR Products Inc. developed untreated knit zirconia cloth typed ZYK-15H in late 1960’s[2]. It has been used in Ni-H2 batteries for several decades. These Ni-H2 batteries offer the distinct advantage that they can be utilized in extreme depth of discharge applications with little or no loss of capacity. In China, the research on fabrication of zirconia separator has been carried out recently[3-6], and some properties of the separators fabricated are as good as those of American products.
In this work, zirconia separators are prepared by precursor process[7-9] in which the regenerated cellulose textiles immersed with zirconium salts are oxidized. In order to optimize the fabrication process, the dominant factors in immersion and the main reactions in heating process are studied. And the primary research on the mechanisms of the fabrication is also carried out.
2 ExperimentalZirconia separators were prepared by precursor process. First, the initial organic polymeric textiles was immersed in zirconium oxychloride (ZrOCl2?8H2O) solutions, in which yttria (Y2O3) was doped as a stabilizer of ZrO2. Second, excess solutions among the individual organic fibers was removed by centrifuging before the solutions drying. Third, the immersed textiles was exposed to NH3 gas. Fourth, the preformed textiles with water was washed for several times until PH is 7. Fifth, the preformed textiles was dried at 50 ℃. At last,the immersed dry textiles was heated in order to remove the organic component and form zirconia separators.
The chemical structure of the precursor textile was investigated by infrared spectroscopy (Thermo Electron Nexus 8700 FTIR spectrometer).
The reactions of the immersed textiles during heat treatment were studied by TG-DSC (Netzsch STA409PC, RAMP 10 ℃/min, argon atmosphere) in order for obtaining an appropriate heat treatment schedule.
The macroscopic appearance and microstructure of the zirconia separators prepared in our work were analyzed by SEM (Hitachi S4800, Japan). The phase analysis was carried out through XRD (with Cu Kα, λ=0.154 056 nm, 40 kV, 200 mA, D/MAX2500, Japan). Chemical composition was analyzed by infrared spectroscope (Thermo Electron Nexus 8700 FTIR spectrometer).
3 Results and discussion3.1 Chemical structure of precursor textile
Viscose textile knitted with continuous spinning bright viscose rayon has been selected as precursor in our work. Viscose fiber is one of regenerated cellulose fibers and composed of cellulose basically. As shown in Fig.1, its chemical composition is some kind of polysaccharide.
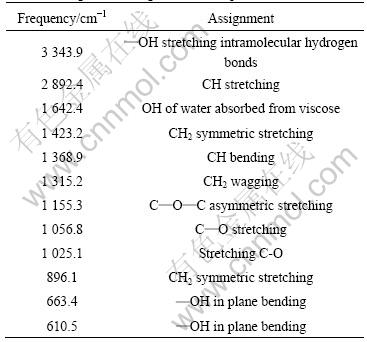
The chemical structure of the viscose textile was determined by infrared spectroscopy (as shown in Fig.2). There are many absorption bands in the spectra and most of them fall into hydroxyl groups[10-12] as listed in Table 1.
Regenerated fibers have a crystalline/amorphous microfibrillar structure. An important factor to the water adsorption property of the fibers is the amount of accessible hydroxyl and carboxyl groups and the portion of amorphous regions where the adsorption process takes place[13]. Compared to other regenerated cellulose fibers, the void volume of viscose is considerably larger and the crystallinity is smaller[13-15]. Thereby viscose has an excellent water adsorption property owing to its large amount of hydroxyl groups, large void volume and small crystallinity.
The mechanism of the precursor process appears as follows: Zirconium oxychloride (ZrOCl2?8H2O) and yttria (Y2O3) are dissolved in water forming an aqueous solution of electrolyte. When the precursor is immersed in the solution, it swells and the interstices between the crystallites open. The dissolved zirconium compounds enter the swollen amorphous regions and become trapped in the amorphous regions when water is removed from the immersed precursor. Thereby, the water adsorption property of the precursor is a dominant factor to the amount of zirconium compounds that can be adsorbed in the precursor, and so significantly influences the properties of the zirconia separator prepared. Viscose textile with high water adsorption property is very suitable to be used as precursor for preparing zirconia battery separator.
Table 1 Assignment of significant absorption bands of viscose
3.2 Research on immersion process
The area densities of the samples prepared by different immersion processes are listed in Table 2.
The area density of sample 4 is the highest among the area densities of the samples owing to the higher solution concentration, the higher immersion temperature, the longer immersion time and the pre-swelling process. While the area density of sample 5 is very low because of the low solution concentration. The area density of sample 2 is a little higher than that of sample 1 owing to the pre-swelling process, and the area densities of sample 2 and sample 3 are similar though the immersion time of sample 3 has been doubled.
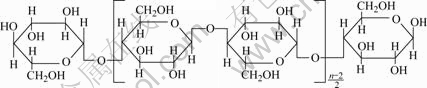
Fig.1 Chemical composition of viscose
Table 2 Area densities of samples prepared by different immersion processes
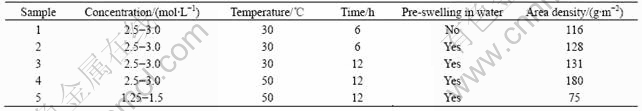
Fig.2 Infrared spectra of viscose textile
Table 2 indicates that the area density of the zirconia separators is influenced by the concentration of zirconium oxychloride solution, the immersion time, the immersion temperature and the pre-swelling process. The concentration and the temperature are the most significant factors in immersion process. With the increase of the solution concentration and the immersion temperature, the area densities of zirconia separators increase.
3.3 Chemical and physical reactions during heat treatment
TG-DSC curves of initial viscose textile and the textile immersed in the solution of zirconium and yttrium compounds are shown in Fig.3. Several processes associated with the water desorption and polymer decomposition were studied.
Below 150 ℃, an endothermic process was detected, with an endothermic peak occurring at approximately 80 ℃ in both DSC curves which corresponds to a mass loss in TG curves. This process is associated with the desorption of the water absorbed caused by the hydrophilic behavior of viscose. The immersed textile has a larger mass loss than that of the initial textile, because both the free water and the crystal water of the zirconium oxychloride absorbed from the solution depart from the immersed textile, while only the water absorbed from air departs from the initial textile.
Moreover, two endothermic peaks appear between 250-450 ℃ corresponding to the decomposition and carbonization of viscose. There is a little difference between the peak temperatures in the two curves. This may be induced by the existence of the zirconium oxychloride in the immersed textile[16]. There is also a mass loss in TG curves in the same period. The main mass loss of initial textile takes place at about 350 ℃, and from then on the mass loss continues but with a lower gradient. While the main mass loss of the immersed textile takes place at about 300 ℃, and there is another mass loss subsequently being related to the decomposition of zirconium compounds and the formation of zirconia.
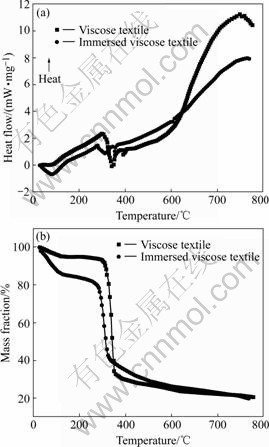
Fig.3 TG—DSC curves of viscose textile and viscose textile immersed in solution of zirconium oxychloride and yttria: (a) DSC curves; (b) TG curves
Intensive decomposition or even ignition of viscose textile may occur if the heating rate is too fast during this process. This will lead to many flaws in the surfaces of the zirconia fibers prepared. Besides, zirconium compounds begin to decompose and the zirconia begins to nucleate during 400-500 ℃. It is known that fine and homogeneous zirconia crystals are beneficial to improving mechanical properties of zirconia separators. With the aim of reducing flaws in the fibers and improving the mechanical properties we should control the heating rate or hold for a period of time at appropriate temperatures during this process. Above 500℃, organic materials have been carbonized and volatilized and substantially all of the zirconium compounds has been converted into zirconia, indicating that the chemical reaction has finished and phase transformation process has began. Hereafter, the temperature can be increased rapidly to the final sintering temperature. After all the steps, zirconia battery separators are prepared.
3.4 Microstructure, phase, chemical composition and alkaline absorption properties of zirconia separators
The macroscopic appearance and microstructure of the zirconia battery separators prepared are shown in Fig.4. The zirconia battery separators are white and very soft. They have the same morphology with the initial viscose textiles.

Fig.4 Macroscopic appearance (a) and microstructure (b) of zirconia separators
The XRD pattern of the zirconia separators is shown in Fig.5. The main phase of the separator is tetragonal zirconia.
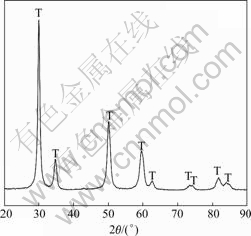
Fig.5 XRD pattern of zirconia separators
The infrared spectra of the zirconia separators is shown in Fig.6, indicating that the organic viscose, as precursor, was almost burn out and the separators are entirely composed of zirconia.
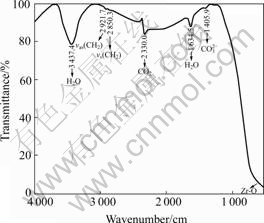
Fig.6 Infrared spectra of zirconia separators
The rate and the amount of alkaline absorption of the zirconia battery separators were tested by 40% KOH solution to be about 5 cm/min and 220% respectively.
4 Conclusions1) Viscose textile is very suitable to be used as the precursor for preparing zirconia battery separators owing to their super water adsorption property.
2) The solution concentration and the temperature are the most important factors in immersion. With the increase of the solution concentration and the immersion temperature, the area densities of zirconia separators increase.
3) With the aim of reducing flaws in the fibers and obtaining fine and homogeneous crystals, one should control the heating rate or hold for a period of time at appropriate temperatures below 500 ℃ in the heat treatment.
4) The main phase of the prepared separators is tetragonal zirconia. The rate and the amount of alkaline absorption of the separators are about 5 cm/min and 220% respectively.
References
[1] SHUKLA A K, VENUGOPALAN S, HARIPRAKASH B. Nickel-based rechargeable batteries [J]. J Power Sources, 2001, 100: 125-148.
[2] HAMLING P, HAMLING B H. Zirconia battery separators—An historical perspective and development update [C]// Battery Conference on Applications and Advances. USA: CA, 1996: 15-23.
[3] LI Hong-zhu, HU Xin-guo, LU-Rong, WU Rong-xian. Study on ZrO2 separator for high pressure Ni-H2 battery [J]. Chinese J Power Sources, 2004, 2: 75-77.
[4] HU Li-ming, CHEN Hong, MA Jun-feng, WU Jian-feng. Study on zirconia cloth [J]. Bulletin of the Chinese Ceramic Society, 2002, 1: 21-24.
[5] HU Li-ming. Study on zirconia cloth [D]. Wuhan: Wuhan University of Technology, 2002: 6-24.
[6] JIA Guang-yao, GUO Zhi-meng, WANG Yao-ming, LUO Wen-hui, QI Jian-mei, HU Li-ming. Preparation of zirconia fiber-film for satellite battery [J]. Bulletin of the Chinese Ceramic Society, 2004, 5: 20-23.
[7] HAMLING B H. Process for producing metal oxide fibers, Textiles and Shapes [P]. US 3385915, 1968.
[8] HAMLING B H. Metal oxide fabrics [P]. US 3663182, 1972.
[9] WONG E M. Method for making metal oxide textiles for spectral emitters [P]. US 5837011, 1998.
[10] CARRILLO F, COLOM X, SU?OL J J, SAURINA J. Structural FTIR analysis and thermal characterization of lyocell and viscose-type fibers [J]. European Polymer J, 2004, 40: 2229-2234.
[11] DU Wei-ping. Analysis of the basic structure of bamboo fiber [J]. Shanghai Textile Science & Technology, 2006, 34(6): 7-11.
[12] COLOM X, CARRILLO F. Crystallinity changes in lyocell and viscose-type fibres by caustic treatment [J]. European Polymer J, 2002, 38: 2225-2230.
[13] KLEINSCHEK K S, KREZE T, RIBITSCH V, STRANAD S. Reactivity and electrokinetical properties of different types of regenerated cellulose fibers [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2001, 195: 275-284.
[14] GINDL W, KECKES J. Strain hardening in regenerated cellulose fibres [J]. Composites Science and Technology, 2006, 66: 2049-2053.
[15] ALBERTI A, BERTINI S, GASTALDI G, IANNACCONE N, MACCIANTELLI D, TORRI G, VISMARA E. Electron beam irradiated textile cellulose fibres. ESR studies and derivatisation with glycidyl methacrylate (GMA) [J]. European Polymer J, 2005, 41: 1787-1797.
[16] YERMOLENKO I N, VITYAZ P A, ULYANOVA T M, FYODOROVA I L. Synthesis and sintering of ZrO2 fibres [J]. Sprechsaal, 1985, 118 (4): 323-325.
(Edited by LAI Hai-hui)
Corresponding author: JIANG LI-jun; Tel: +86-10-82241240; Fax: +86-10-82241294; E-mail: jlj@grinm.com


