J. Cent. South Univ. (2016) 23: 52-58
DOI: 10.1007/s11771-016-3048-6
Effect and mechanism of siderite on reverse anionic flotation of quartz from hematite
LUO Xi-mei(罗溪梅)1, YIN Wan-zhong(印万忠)2, WANG Yun-fan(王云帆)3,
SUN Chuan-yao(孙传尧)4, MA Ying-qiang(马英强)2, LIU Jian(刘建)1
1. State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization,
Faculty of Land and Rescource Engineering, Kunming University of Science and Technology,Kunming 650093, China;
2. College of Zijin Mining, Fuzhou University, Fuzhou 350116, China;
3. Faculty of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China;
4. Beijing General Research Institute of Mining & Metallurgy, Beijing 100044, China
 Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Central South University Press and Springer-Verlag Berlin Heidelberg 2016
Abstract:
Reverse flotation technology is one of the most efficient ways to improve the quality and reduce impurity of iron concentrate. Mineral processors dealing with hematite face a challenge that the flotation results of reverse flotation of hematite are poor in presence of siderite using fatty acid as collector, starch as depressant of iron minerals and calcium ion as activator of quartz at strong alkaline pH. In this work, the effect of siderite on reverse anionic flotation of quartz from hematite was investigated. The effect mechanism of siderite on reverse flotation of hematite was studied by solution chemistry, ultraviolet spectrophotometry (UV) and Fourier transform infrared spectroscopy (FTIR). It was observed that siderite had strong depressive effect on quartz in flotation using sodium oleate as collector, corn starch as depressant of iron minerals and calcium chloride as activator of quartz at strong alkaline pH. The starch was adsorbed onto calcium carbonate by chemical reaction which was formed by 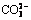 from siderite dissolution and Ca2+ from calcium chloride as activator of quartz and precipitated on the surface of quartz, which resulted in improving the hydrophilic ability of quartz.
from siderite dissolution and Ca2+ from calcium chloride as activator of quartz and precipitated on the surface of quartz, which resulted in improving the hydrophilic ability of quartz.
Key words:
1 Introduction
Existing studies on the flotation of iron ores have shown that iron ores can be floated with both anionic and cationic collectors [1-7]. However, the flotabilities of iron ore minerals such as hematite and siderite are always lower than that of quartz under similar conditions [8-12].
Reverse cationic/anionic flotation technology is one of the most efficient ways to improve the quality and reduce impurity of iron concentrate [13-14]. During the flotation process, quartz is attached to air bubble and floats upward into a froth layer leaving hematite as the non-floated fraction. Iron minerals are generally depressed with starch [15-19]. In most iron ore beneficiation plants, amine is generally used as collector of quartz and starch is generally used as depressant of iron minerals [7, 20]. In china, fatty acid is often employed as collector, calcium ion is generally employed as activator of quartz and starch is often employed as depressant of iron minerals according to iron ore characteristic and technological conditions [21-23].
In recent years, mineral processors dealing with hematite have faced a challenge that the flotation results of reverse flotation of hematite are poor in presence of siderite using fatty acid as collector, starch as depressant of iron minerals and calcium ion as activator of quartz at strong alkaline pH [24-25]. The Fe grade in non-floated product decreases with increasing the siderite content in iron ores. However, researchers have not been able to find the exact reasons for the phenomenon. In this work, the pure mineral flotation tests were conducted using sodium oleate as collector, corn starch as depressant of iron minerals and calcium chloride as activator of quartz to discuss the effect of siderite on reverse flotation of hematite. this work aimed to investigate the effect mechanism of siderite on reverse flotation of hematite by solution chemistry, ultraviolet spectrophotometry (UV) and Fourier transform infrared spectroscopy (FTIR).
2 Materials and methods
2.1 Materials and reagents
The siderite sample and hematite sample were obtained from Anshan city, Liaoning province, China. The quartz sample was purchased from Yingkou city, Liaoning province, China. The three samples were carefully ground in a ball mill, processed by gravity concentration and sieved to (-106+45) μm size fractions. X-ray diffraction analysis and chemical analysis of three samples showed that the siderite, hematite and quartz samples were of high purity. The siderite sample contained about 44.65% Fe (mass fraction), the hematite sample contained about 67.45% Fe (mass fraction), and the quartz sample had a purity of 99.77% SiO2 (mass fraction).
Sodium oleate with 98% purity was used as the anionic collector. Calcium chloride with 99% purity was used as the activator of quartz. Corn starch was employed as the depressant of siderite and hematite, which was dissolved in distilled water by adding 20% NaOH by weight at 50 °C on a hot plate. Analytical grade NaOH was used for pH adjustment to keep the pH at about 11.4. Distilled water was used in all experiments.
2.2 Method
Flotation experiments were carried out in a 30 mL flotation cell with impeller speed of 1500 r/min. A 2.0 g (2.7 g) sample of particle size (-106+45) μm composed of quartz, hematite and siderite in different proportions by mass according to test design was mixed with 25 mL distilled water in flotation cell for 1 min. Then, NaOH was added to keep the pH at about 11.4 and the pulp was conditioned for 2 min. And then, calcium chloride, starch, sodium oleate were added separately and the pulp was conditioned for 2 min with each reagent, respectively. The flotation time was fixed for 3 min at room temperature (25 °C). After flotation, the floated product and non-floated product were collected, filtered, dried, weighed and analyzed, respectively.
The sodium oleate adsorption capacity adsorbed onto minerals was measured by ultraviolet spectrophotometer with the type of Spectro Flex 6600. The steps that have been taken are similar to those of Ref. [26].
Perkin Elmer Spectrum One FT-IR Spectrometer had been used to understand the reagent-mineral interaction with the type of Nicolet380 FT-IR.
3 Results and discussion
3.1 Effect of siderite on flotation separation of hematite and quartz
Figure 1 shows the quartz recovery and Fe grade in non-floated product as a function of percentage of siderite in mixed minerals using sodium oleate as collector, corn starch as depressant of iron minerals and calcium chloride as activator of quartz at pH 11.4. As can be seen, the Fe grade in non-floated product strongly decreases, from 54.15% to below 40%. Meanwhile, the quartz recovery in non-floated product significantly increases, from 19.50% to 98.00%. That means that siderite has strong negative impact on reverse flotation of hematite.
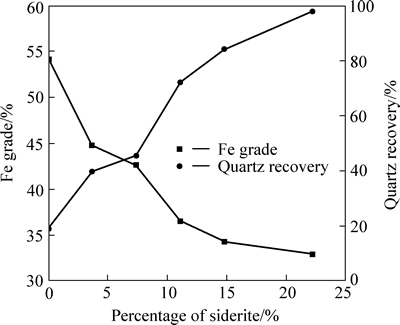
Fig. 1 Quartz recovery and Fe grade in non-floated product as a function of percentage of siderite (sodium oleate dosage: 160 mg/L; corn starch dosage: 60 mg/L; calcium chloride dosage: 100 mg/L; pH:11.40; a 2.7 g sample: composed of siderite, hematite and quartz, proportion by mass of hematite and quartz: 4:5)
3.2 Effect of siderite and hematite on quartz flotation
The results shown in Fig. 2 indicate that both the SiO2 grade and quartz recovery in floated products slightly decreases with increasing the percentage of hematite. That means hematite may have little impact on quartz flotation using sodium oleate, corn starch and calcium chloride at strong alkaline pH.
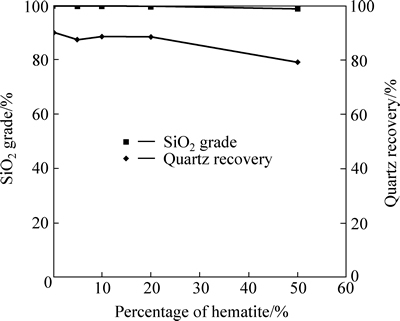
Fig. 2 SiO2 grade and quartz recovery in floated product as a function of percentage of hematite (sodium oleate dosage: 160 mg/L; corn starch dosage: 60 mg/L; calcium chloride dosage: 100 mg/L; pH: 11.40; a 2.0 g sample: composed of hematite and quartz in different proportions by mass)
Figure 3 shows SiO2 grade and quartz recovery in floated product as a function of percentage of siderite by mass. It is very clearly seen that as far as the percentage of siderite increases, the quartz recovery in floated product decreases sharply. When the percentage of siderite is only 5%, the quartz recovery is reduced from 90.05% to 44.88%. The results are consistent with observations reported by Zhang et al [24] and Yang [25].
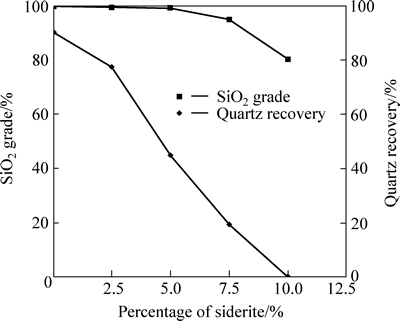
Fig. 3 SiO2 grade and quartz recovery in floated product as a function of percentage of siderite (sodium oleate dosage: 160 mg/L; corn starch dosage: 60 mg/L; calcium chloride dosage: 100 mg/L; pH: 11.38; a 2.0 g sample: composed of siderite and quartz in different proportions by mass)
The results shown in Figs. 2 and 3 stress the role that the cause why siderite can deteriorate the reverse flotation of hematite is due to the passive impact of siderite on quartz flotation.
3.3 Effect of sodium oleate dosage on quartz flotation from quartz-siderite mixed minerals
From Table 1, it can be seen that the quartz recovery in floated product slightly increases and the sodium oleate adsorption capacity on minerals changes a little when sodium oleate dosage increases from 160 mg/L to 240 mg/L. That means that the main cause why siderite has strong negative impact on quartz flotation has little relationship to sodium oleate consumption by siderite.
3.4 Effect of reagent type on quartz flotation from quartz-siderite mixed minerals
The quartz recovery in floated product in presence and absence of corn starch is listed in Table 2. It can be seen clearly that in anionic flotation of quartz using sodium oleate, corn starch and calcium chloride at strong alkaline pH, when the percentage of siderite increases from 0% to 5%, the quartz recovery in floated product reduces from 93.80% to 62.20%. However, in absence of corn starch, the quartz recovery increases from 62.20% to 88.32%. That illustrates that siderite has little impact on quartz flotation in absence of corn starch, and has significant impact on quartz flotation in presence of corn starch. Therefore, it can be concluded that the cause why siderite has strong passive impact on quartz flotation is mainly due to corn starch.
It can be seen from Table 3 that the sodium oleate adsorption capacity on mixed minerals (siderite 0.2 g, quartz 1.8 g) in presence of sodium oleate, calcium chloride and starch significantly decreases from 1.63 mg/g to 0.26 mg/g in contrast with in absence of starch. That illustrates that when starch and siderite both exist, the adsorption of sodium oleate onto quartz is baffled. However, it can also be seen that when the percentage of siderite is 0%, the sodium oleate adsorption capacity on quartz in presence of sodium oleate, calcium chloride and starch only changes a little in contrast with in absence of starch. These results are consistent with the research reported by JIANG et al [26] and MA [27], who reported that starch had little impact on quartz activated by Ca2+ in anionic flotation. From the results above, it can be calculated that the performance that starch has depressing effect on quartz activated by Ca2+ in flotation is correlated with siderite. Only when siderite is present, starch can have depressing effect on quartz in flotation. Therefore, the passive impact of siderite on quartz in flotation is caused by both starch and siderite.
3.5 Effect of siderite dissolution on quartz flotation
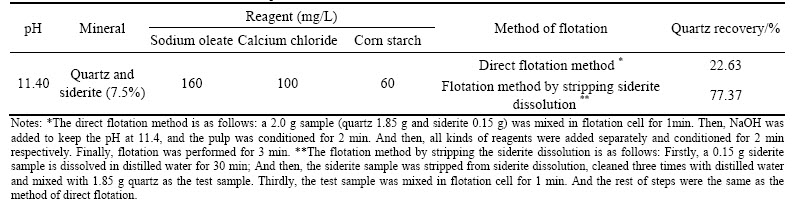
The results in Table 4 show that siderite dissolution has an even greater impact on quartz flotation. The quartz recovery in floated product is greatly improved by stripping the siderite dissolution in flotation.
It is shown by the data [29-30] siderite is easily dissolved in water. The higher the pH is, the easier the siderite dissolves. In reverse anionic flotation of iron ores, calcium ion is often added to activate quartz, which is in favor of siderite dissolution. The dissolved components logarithm diagram of siderite shows that there is a lot of  ion in siderite dissolution at strong alkaline pH. Therefore, when there is a lot of
ion in siderite dissolution at strong alkaline pH. Therefore, when there is a lot of  calcium ion composition is complicated. Calcium ion in sodium carbonate system may be the following reaction. The principle reaction of calcium ion in sodium carbonate system is as follows [31-32]:
calcium ion composition is complicated. Calcium ion in sodium carbonate system may be the following reaction. The principle reaction of calcium ion in sodium carbonate system is as follows [31-32]:

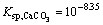 (1)
(1)
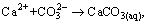
 (2)
(2)

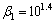 (3)
(3)
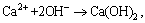
 (4)
(4)
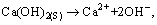
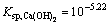 (5)
(5)
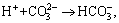
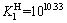 (6)
(6)

 (7)
(7)
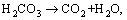
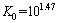 (8)
(8)
Table 1 Quartz recovery and sodium oleate adsorption capacity on mineral as a function of sodium oleate dosage
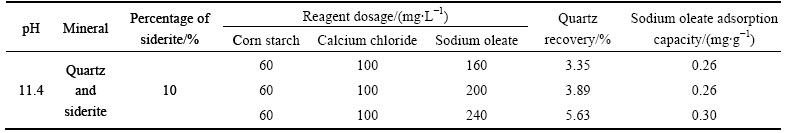
Table 2 SiO2 grade and quartz recovery in floated product as a function of reagent types in presence and absence of siderite
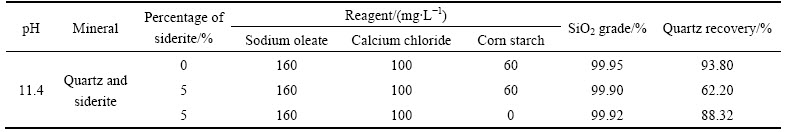
Table 3 Sodium oleate adsorption capacity on quartz as a function of corn starch in presence and absence of siderite
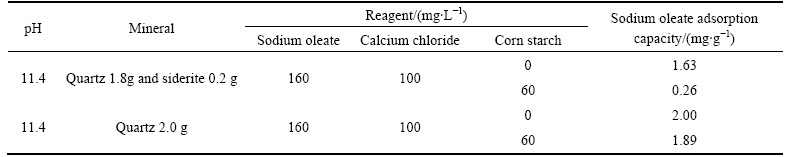
Table 4 Influence of siderite dissolution on quartz flotation
Different composition concentrations of calcium ion in balance system of sodium carbonate as a function of pH are shown in Fig.4 according to the Eqs. (1) to (8). Fig.4 shows that calcium ion exists in the form of Ca2+ when pH is below 9.98, and in the form of CaCO3 precipitation when pH is above 9.98. Because there is much  from siderite dissolution at pH 11.4, when calcium ion is added as activator of quartz, it will exist on the surface of quartz in the form of CaCO3 precipitation, which makes the surface of quartz have the same properties as calcium carbonate. In other words, the flotation characteristics of quartz are similar to calcite.
from siderite dissolution at pH 11.4, when calcium ion is added as activator of quartz, it will exist on the surface of quartz in the form of CaCO3 precipitation, which makes the surface of quartz have the same properties as calcium carbonate. In other words, the flotation characteristics of quartz are similar to calcite.
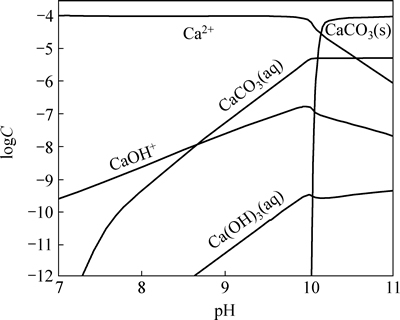
Fig. 4 Species distribution of Ca2+ in balance system of sodium carbonate (Concentration of Ca2+: 1.0×10-4 mol/L)
3.6 Effect of carbonate ion and starch on quartz flotation
In order to verify both  dissolved from siderite and starch lead to reduction of quartz recovery in quartz flotation, quartz recovery as a function of sodium carbonate dosage and starch dosage are shown in Figs. 5 and 6, respectively.
dissolved from siderite and starch lead to reduction of quartz recovery in quartz flotation, quartz recovery as a function of sodium carbonate dosage and starch dosage are shown in Figs. 5 and 6, respectively.
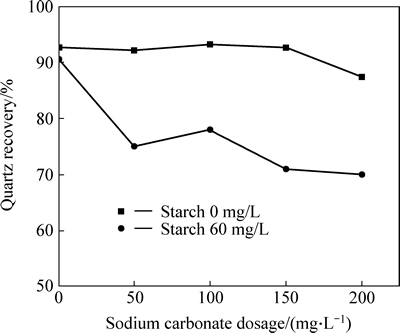
Fig. 5 Quartz recovery as a function of sodium carbonate dosage in presence and absence of starch (sodium oleate dosage: 160 mg/L; calcium chloride dosage: 100 mg/L; pH: 11.38; a 2.0 g sample composed of quartz)
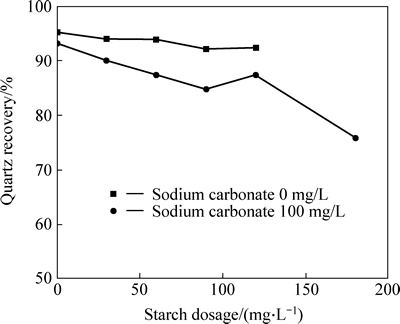
Fig. 6 Quartz recovery as a function of starch dosage in presence and absence of sodium carbonate (sodium oleate dosage: 160 mg/L; calcium chloride dosage: 100 mg/L; pH: 11.38; a 2.0 g sample composed of quartz)
The results from Figs. 5 and 6 indicate that starch has weak impact on quartz recovery in flotation in absence of  and has strong negative impact on quartz recovery in presence of
and has strong negative impact on quartz recovery in presence of 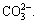 Therefore, it can be seen that both
Therefore, it can be seen that both 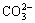 dissolved from siderite and starch have strong passive impact on quartz recovery in flotation. And it can be speculated the reason of that is the starch has strong depressive effect on quartz covered by calcium carbonate in flotation.
dissolved from siderite and starch have strong passive impact on quartz recovery in flotation. And it can be speculated the reason of that is the starch has strong depressive effect on quartz covered by calcium carbonate in flotation.
3.7 FTIR studies of minerals before and after reaction with different reagents
3.7.1 FTIR spectra of calcium carbonate before and after reaction with starch
The FTIR spectra of calcium carbonate before and after reaction with starch are shown in Figs. 7(a) and (b). It can be seen clearly from Fig. 7(b) that a new peak at 1620.91 cm-1 appears due to interaction with starch. And the peak at 3445.56 cm-1 may be the result of the shift of the peak at 3431.44 cm-1 attributed to stretching vibrations of hydroxyl group owing to some chemical bonding with starch. The peak at 1408.09 cm-1 may be the result of the shift of the peak at 1421.53 cm-1 due to asymmetric stretching vibrations of C—O groups. On the basis of FTIR spectra, it is clear that chemical interaction occurs between calcium carbonate and starch. It is also clear from FTIR spectra that starch is specifically adsorbed onto calcium carbonate. That is accordance with the foregoing analysis. These results are consistent with the research reported by Li et al [33] and Pinto et al [34], who reported that starch had strong depressing effect on calcite (CaCO3) in flotation owing to the chemical interaction between starch and calcite.
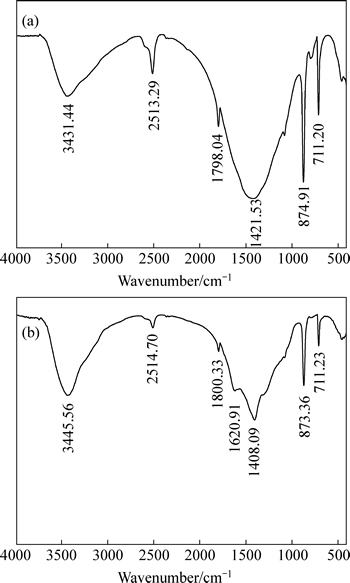
Fig. 7 Infrared spectra of calcium carbonate before (a) and after (b) reaction with starch
3.7.2 FTIR spectra of quartz before and after treatment with different reagents
In order to reaffirm that the starch is adsorbed onto quartz attributing to calcium carbonate on the surface of quartz, the FTIR spectra of quartz before and after treatment with calcium chloride and starch, and with sodium carbonate, calcium chloride and starch are shown in Fig. 8.
Figures 8(a) and (b) show that there is no new peak on quartz after treatment with calcium chloride and starch. That means the adsorption of starch onto quartz is very weak. A possible explanation is starch in absence of 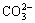 is adsorbed onto quartz through CaOH+ only by the electrostatic attraction and hydrogen-bond interaction, whose acting force is very weak [27].
is adsorbed onto quartz through CaOH+ only by the electrostatic attraction and hydrogen-bond interaction, whose acting force is very weak [27].
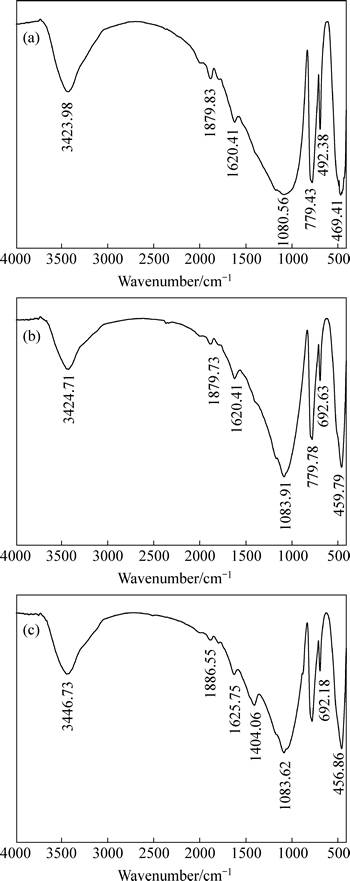
Fig. 8 Infrared spectra of quartz before treatmeat (a), after treatment with calcium chloride and starch (b) and with sodium carbonate, calcium chloride and starch (c)
As can be seen from Figs. 8(a) and (c), a new peak at 1404.06 cm-1 appears on the spectrum of quartz after treatment with sodium carbonate, calcium chloride and starch. The peak at 1404.06 cm-1 is due to bending vibrations of CH2 group of starch. According to the results from Fig. 7(b) and Fig. 8(c), the new peak is just attributed to the chemical adsorption of starch onto calcium carbonate. Meanwhile, the peak at 3423.98 cm-1 shifts to the peak at 3446.73 cm-1, which is due to stretching vibrations of hydroxyl group of starch. The results above indicate that chemical reaction between starch and quartz in presence of sodium carbonate and calcium chloride occurs.
In conclusion, it can be seen from the results above that the reason siderite has strong negative effect on quartz flotation at strong alkaline pH is that starch is adsorbed onto calcium carbonate by chemical reaction which is formed by 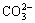 from siderite dissolution and Ca2+ from calcium chloride as activator of quartz and precipitates on the surface of quartz, which results in improving the hydrophilic ability of quartz.
from siderite dissolution and Ca2+ from calcium chloride as activator of quartz and precipitates on the surface of quartz, which results in improving the hydrophilic ability of quartz.
4 Conclusions
1) Siderite has strong negative impact on flotation separation of hematite and quartz using sodium oleate as collector, corn starch as depressant of iron minerals and calcium chloride as activator of quartz at pH 11.4. The quartz recovery in non-floated product significantly increases along with the percentage of siderite. The reason of that is siderite has strong passive effect on quartz flotation.
2) That siderite has strong negative impact on quartz flotation using sodium oleate as collector, corn starch as depressant of iron minerals and calcium chloride as activator of quartz at strong alkaline pH is irrelevant to sodium oleate consumption by siderite and is concerned with starch and 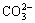 from siderite dissolution. The starch is adsorbed onto calcium carbonate by chemical reaction which is formed by
from siderite dissolution. The starch is adsorbed onto calcium carbonate by chemical reaction which is formed by 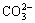 from siderite dissolution and Ca2+ from calcium chloride as activator of quartz and precipitates on the surface of quartz, which results in improving the hydrophilic ability of quartz.
from siderite dissolution and Ca2+ from calcium chloride as activator of quartz and precipitates on the surface of quartz, which results in improving the hydrophilic ability of quartz.
References
[1] SUN Bing-quan. Progress in china’s beneficiation technology for complex refractory iron ore [J]. Metal Mine, 2005, 8: 31-34. (in Chinese)
[2] UWADIALE G G O O. Flotation of iron oxides and quartz [J]. A review. Mineral Processing and Extractive Metallurgy Review, 1992, 11(3): 129-161.
[3] PAPINI R M, BRAND O P R G, PERES A E C. Cationic flotation of iron ores: amine characterization and performance [J]. Minerals and Metallurgical Processing, 2001, 18(1): 5-9.
O P R G, PERES A E C. Cationic flotation of iron ores: amine characterization and performance [J]. Minerals and Metallurgical Processing, 2001, 18(1): 5-9.
[4] ARAUJO A C, VIANA P R M, PERES A E C. Reagents in iron ores flotation [J]. Minerals Engineering, 2005, 18(2): 219-224.
[5] MOWLA D, KARIMI G, OSTADNEZHAD K. Removal of hematite from silica sand ore by reverse flotation technique [J]. Separation and Purification Technology, 2008, 58(3): 419-423.
[6] LIMA N P, VALAD O G E S, PERES A E C. Effect of amine and starch dosage on the reverse cationic flotation of an iron ore [J]. Minerals Engineering, 2013, 45: 180-184.
O G E S, PERES A E C. Effect of amine and starch dosage on the reverse cationic flotation of an iron ore [J]. Minerals Engineering, 2013, 45: 180-184.
[7] FILIPPOV L O, SEVEROV V V, FILIPPOVA I V. An overview of the beneficiation of iron ores via reverse cationic flotation [J]. International Journal of Mineral Processing, 2014, 127: 62-69.
[8] IWASAKI I. Iron ore flotation. theory and practice [J]. Minerals Engineering, 1983, 35(6):622-631.
[9] MONTES-SOTOMAYOR S, HOUOTR, KONGOLO M. Flotation of silicate gangue iron ores: mechanism and effect of starch [J]. Minerals Engineering, 1998, 11(1): 71-76.
[10] WANG Yu-hua, REN Jian-wei. The flotation of quartz from iron minerals with a combined quaternary ammonium salt [J]. International Journal of Mineral Processing, 2005, 77(2): 116-122.
[11] BIRINCI M, MILLER J D, SARIKAYA M, WANG Xu-ming. The effect of an external magnetic field on cationic flotation of quartz from magnetite [J]. Minerals Engineering, 2010, 23(10): 813-818.
[12] HUANG Zhi-qiang, ZHONG Hong, WANG Shuai, XIA Liu-yin, ZOU Wei-bo, LIU Guang-yi. Investigations on reverse cationic flotation of iron ore by using a Gemini surfactant: ethane-1,2-bis (dimethyl-dodecyl-ammonium bromide) [J]. Chemical Engineering Journal, 2014, 257: 218-228.
[13] MA X, MARQUES M, GONTIJO C. Comparative studies of reverse cationic/anionic flotation of Vale iron ore [J]. International Journal of Mineral Processing, 2011, 100(3/4): 179-183.
[14] TURRER H D G, PERES A E C. Investigation on alternative depressants for iron ore flotation [J]. Minerals Engineering, 2010, 23(11/12/13): 1066-1069.
[15] LIU Ai-guo, WU Rong-chang, ESCHENAZI E, PAPADOPOULOS K. AFM on humic acid adsorption on mica [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2000, 174(1): 245-252.
[16] MATTEDI V A, OLIVEIRA J F. Adsorption of starch onto apatite and magnetite and their selective flotation [J]. In: VI Southern Hemisphere Meeting on Mineral Technology, Rio de Janeiro, 2001, 1: 271-274.
[17] PAVLOVIC S, BRAND O P R G. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz [J]. Minerals Engineering, 2003, 16(11): 1117-1122.
O P R G. Adsorption of starch, amylose, amylopectin and glucose monomer and their effect on the flotation of hematite and quartz [J]. Minerals Engineering, 2003, 16(11): 1117-1122.
[18] NANTHAKUMAR B, GRIMM D, PAWLIK M. Anionic flotation of high-iron phosphate ores—Control process water chemistry and depression of iron minerals by starch and guar gum [J]. International Journal of Mineral Processing, 2009, 92(1/2): 49-57
[19] KAR B, SAHOO H, RATH S S, DAS B. Investigations on different starches as depressants for iron ore flotation [J]. Minerals Engineering, 2013, 49: 1-6.
[20] LIMA R M F, BRAND O P R G, PERES A.E.C. The infrared spectra of amine collectors used in the flotation of iron ores [J]. Minerals Engineering, 2005, 18(2): 267-273.
O P R G, PERES A.E.C. The infrared spectra of amine collectors used in the flotation of iron ores [J]. Minerals Engineering, 2005, 18(2): 267-273.
[21] CHEN Wen. Technological progress in processing low-grade fine-grained complicated refractory iron ores [J]. Metal mine, 2010, 5: 55-59, 80. (in Chinese)
[22] LUO Xi-mei, YIN Wan-zhong, YAO Jin, SUN Chuan-yao, CAO Yang, MA Ying-qiang, HOU Ying. Flotation separation of magnetic separation concentrate of refractory hematite containing carbonate with enhanced dispersion [J]. The Chinese Journal of Nonferrous Metals, 2013, 23(1): 238-245. (in Chinese)
[23] LIU Jie, ZHOU Ming-shun, ZHAI Li-wei, LIU Jiong-tian, CAO Yi-jun. Present status of china’s complex refractory iron ore study [J]. China mining magazine, 2011, 20(5): 63-66. (in Chinese)
[24] ZHANG Ming, LV Zhen-fu, YIN Wan-zhong, HAN Yue-xin. Influence of the siderite in donganshan iron ore on reverse flotation [J]. Metal mine, 2007, 9: 62-64. (in Chinese)
[25] YANG Bin. Study on separation technology and mechanism of siderite and hematite [D]. Changsha: Central South University, 2010. (in Chinese)
[26] JIANG Hao, LIU Guo-rong, Hu Yue-hua, XU Long-hua, YU Wa-wen, XIE Zhen, CHEN Hao-chuan. Flotation and adsorption of quaternary ammonium salts collectors on kaolinite of different particle size [J]. International Journal of Mining Science and Technology, 2013, 23(2): 249-253.
[27] MA Song-bo. Froth flotation of hematite with starch as depressant [D]. Shenyang: North East University, 2006. (in Chinese)
[28] YE H, Matsuoka I. Reverse flotation of fine quartz from dickite with oleate [J]. International Journal of Mineral Processing, 1993, 40(1/2): 123-136.
[29]  P, DANDURAND J L, HARRICHOURY J C. Solubility product of siderite (FeCO3) as a function of temperature (25-250 °C) [J]. Chemical Geology, 2009, 265(1/2): 3-12.
P, DANDURAND J L, HARRICHOURY J C. Solubility product of siderite (FeCO3) as a function of temperature (25-250 °C) [J]. Chemical Geology, 2009, 265(1/2): 3-12.
[30] MARTA M,  B, GUILLAUME F, FRANCOIS G. In-situ monitoring of the formation of carbon compounds during the dissolution of iron (Ⅱ) carbonate (siderite) [J]. Chemical Geology, 2011, 290(3/4): 145-155.
B, GUILLAUME F, FRANCOIS G. In-situ monitoring of the formation of carbon compounds during the dissolution of iron (Ⅱ) carbonate (siderite) [J]. Chemical Geology, 2011, 290(3/4): 145-155.
[31] YANG Shao-yan. Study on Theory and process of flotation of calamine [D]. Changsha: Central South University, 2010. (in Chinese)
[32] TANG Pei-hui. Selective Flotation between apatite and siliceous gangue [D]. Changsha: Central South University, 2011. (in Chinese)
[33] Li Ye, SOMASUNDARAN P, PINTO C L L. Adsorption properties and interaction mechanism of starch-type polysaccharides onto fluorite and calcite [J]. Nonferrous Metals, 1996, 48(1): 26-30.
[34] PINTO C L L, ARAUJO A C, PERES A E C. The effect of starch, amylose and amylopectin on the depression of oxi-minerals [J]. Minerals Engineering, 1992, 5(3): 469-478.
(Edited by DENG Lü-xiang)
Foundation item: Project (51374079) supported by the National Natural Science Foundation of China
Received date: 2014-09-12; Accepted date: 2015-01-31
Corresponding author: YIN Wan-zhong, Professor, PhD; Tel: +86-24-83673958; E-mail: yinwanzhong@mail.neu.edn.cn
Abstract: Reverse flotation technology is one of the most efficient ways to improve the quality and reduce impurity of iron concentrate. Mineral processors dealing with hematite face a challenge that the flotation results of reverse flotation of hematite are poor in presence of siderite using fatty acid as collector, starch as depressant of iron minerals and calcium ion as activator of quartz at strong alkaline pH. In this work, the effect of siderite on reverse anionic flotation of quartz from hematite was investigated. The effect mechanism of siderite on reverse flotation of hematite was studied by solution chemistry, ultraviolet spectrophotometry (UV) and Fourier transform infrared spectroscopy (FTIR). It was observed that siderite had strong depressive effect on quartz in flotation using sodium oleate as collector, corn starch as depressant of iron minerals and calcium chloride as activator of quartz at strong alkaline pH. The starch was adsorbed onto calcium carbonate by chemical reaction which was formed by 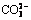 from siderite dissolution and Ca2+ from calcium chloride as activator of quartz and precipitated on the surface of quartz, which resulted in improving the hydrophilic ability of quartz.
from siderite dissolution and Ca2+ from calcium chloride as activator of quartz and precipitated on the surface of quartz, which resulted in improving the hydrophilic ability of quartz.


