Trans. Nonferrous Met. Soc. China 26(2016) 1155-1162
High temperature oxidation behavior of Ti(Al,Si)3 diffusion coating on γ-TiAl by cold spray
Ji-qiang WANG, Ling-yan KONG, Tie-fan LI, Tian-ying XIONG
Institute of Metal Research, Chinese Academy of Sciences, Shenyang 110016, China
Received 26 May 2015; accepted 19 October 2015
Abstract:
A Ti(Al,Si)3 diffusion coating was prepared on γ-TiAl alloy by cold sprayed Al-20Si alloy coating, followed by a heat-treatment. The isothermal and cyclic oxidation tests were conducted at 900 °C for 1000 h and 120 cycles to check the oxidation resistance of the coating. The microstructure and phase transformation of the coating before and after the oxidation were studied by SEM, XRD and EPMA. The results indicate that the diffusion coating shows good oxidation resistance. The mass gain of the diffusion coating is only a quarter of that of bare alloy. After oxidation, the diffusion coating is degraded into three layers: an inner TiAl2 layer, a two-phase intermediate layer composed of a Ti(Al,Si)3 matrix and Si-rich precipitates, and a porous layer because of the inter-diffusion between the coating and substrate.
Key words:
Ti(Al,Si)3 diffusion coating; γ-TiAl alloy; cold spray; heat-treatment; high temperature oxidation;
1 Introduction
TiAl-based alloys exhibit great potential for high-temperature structural applications such as turbine blades of internal-combustion engines, owing to their low density, high specific strength, and high creep resistance [1-3]. However, the poor oxidation resistance at temperature above 800 °C and environmental embrittlement of γ-TiAl limits its application [4-6]. Therefore, the γ-TiAl-based alloys should be protected in order to be used at elevated temperatures.
Many coating systems were applied to improving the oxidation resistance of γ-TiAl alloy. XU et al [7] reported the NiCrAlY coating with and without an Al interlayer [7]. GAO et al [8] reported the oxidation behavior of γ-TiAl-based alloy with Al2O3-Y2O3 composite coatings. GAUTHIER et al [9] and BRAUN et al [10] studied the performance of thermal barrier coatings on γ-TiAl. IZUMI et al [11] reported the superior long-term oxidation resistance of Ni-Al-coated TiAl alloys. The TiAl3 intermetallic had good compatibility with γ-TiAl substrate and attracted great interest. The most common method to prepare TiAl3 coating was aluminizing, including pack cementation [12], thermal spray aluminum and annealing [13], deposition of aluminum by PVD and annealing [14], electro- deposition aluminum and annealing [15], and cold sprayed aluminum and annealing [16].
However, the brittleness and fast degradation during the high temperature exposure decreased the oxidation resistance of the TiAl3 coating. To improve the properties of TiAl3, the coating modified with the third element was widely reported [17-22]. XIONG et al [17,18] reported liquid-phase siliconized by Al-Si alloys on TiAl-based alloy and studied its oxidation resistance. SWADZBA et al [19] studied the long-term cyclic oxidation of Al-Si diffusion coatings deposited by arc-PVD. XIANG et al [20] studied co-deposition of Al and Si to form oxidation-resistant coatings by pack cementation. GORAL et al [21,22] studied the influence of Si on structure and oxidation resistance of aluminide coatings. LI et al [23] reported the microstructure and high temperature oxidation resistance of Si-Y co-deposition coatings prepared on TiAl alloy by pack cementation process. The results showed that the aluminide coating modified with silicon could improve the oxidation resistance of the bare alloy significantly.
In the study of joining pure aluminum to the TiAl alloy by using a Si-containing aluminum-based brazing alloy, XIONG et al [24] found that Si diffused strongly from the brazing alloy to the surface of TiAl, indicating that Si also has a great affinity with Ti in TiAl. Taking the above phenomenon into account, a Si-modified Ti(Al,Si)3 coating was firstly deposited on γ-TiAl alloy by cold sprayed Al-20Si alloy coating and subsequent heat-treatment in this work. The high temperature oxidation resistance of the coating was investigated. The phase transformation, microstructure and composition of the coating were also studied.
2 Experimental
The nominal composition of the γ-TiAl substrate was Ti-47Al-2Cr-2Nb-0.15B (mole fraction, %), which was provided by Titanium Alloys Division, Institute of Metal Research, Chinese Academy of Sciences. The ingot was cut into 15 mm × 10 mm × 2 mm coupons. The surface of all the specimens was ground with SiC paper up to 800 grit, cleaned ultrasonically in anhydrous alcohol, dried and then pilled before cold spray.
Commercial Al-20Si (mass fraction, %) powders (by Changsha Tianjiu metallic material Co., Ltd) were used for cold spray. Before spray, the powders were dried at 80 °C for 4 h to remove the moisture. The cold spray device was manufactured by the Institute of Metal Research, Chinese Academy of Sciences. The De Laval nozzle was a rectangle exit equipped with a cross section of 2 mm × 10 mm and a throat of 2 mm × 2 mm. The parameters for the cold spray process were 360 °C, 2.0 MPa, and 15 mm for gas temperature, gas pressure and standoff distance, respectively. The as-sprayed specimens were subjected to heat-treatment at 750 °C for 12 h under argon gas flow of 40 mL/min according to the optimal experiment. The heating rate was 3 °C/min. After the heat-treatment, the furnace was then cooled to room temperature at its natural rate by switching off its power.
The isothermal oxidation tests were performed in air in a muffle furnace at 900 °C for 1000 h. The cyclic oxidation tests were also conducted in a muffle furnace at 900 °C for 120 cycles. The samples were kept at 900 °C for 1 h and cooled down to room temperature for 10 min as a cycle. During the oxidation test, all samples were put into the Al2O3 crucible. Therefore, the exfoliated oxide was collected and the mass of the exfoliated oxide was determined. The mass changes of all samples were measured at regular intervals with a balance. The sensitivity of the balance is 10-5 g.
X-ray diffraction (XRD) analysis was conducted on D/max-2500pc (RIGAKU, Japan). Scanning electron microscopy (SEM-EDS) imaging was carried out using JSM-6301F (SHIMADZU, Japan) and Inspect F50 (FEI, USA). The electron probe microanalysis was performed using EPMA-1610 (SHIMADZU, Japan).
3 Results and discussion
3.1 Microstructure of Al-20Si alloy coating before and after heat-treatment
Figure 1 shows the cross-section microstructures of Al-20Si alloy coating before and after the heat- treatment. From Fig. 1(a), it could be seen that the Al-20Si alloy coating was about 50 μm in thickness. It bonded well with the substrate and was compact without visible pores. The Si content in the coating was slightly less than that in the feedstock, which might be caused by the Si particle shedding during the sample grinding and polishing. From the XRD pattern of the Al-20Si alloy coating as shown in Fig. 2(a), the as-sprayed coating was composed of aluminum and silicon. This result indicated that no significant phase transformation occurred during the cold spray process. This is mainly attributed to the low spray temperature.
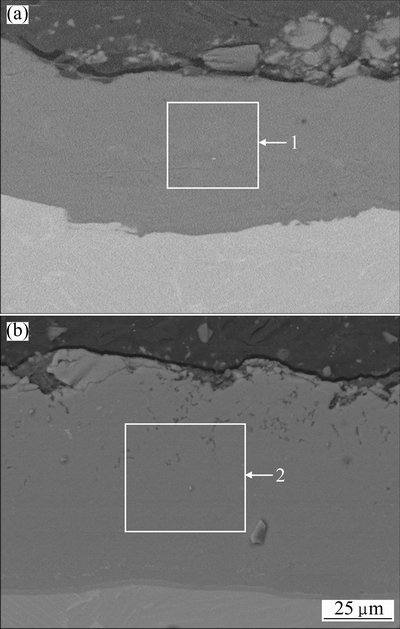
Fig. 1 SEM images of cross-section microstructures of Al-20Si alloy coating before (a) and after (b) heat-treatment
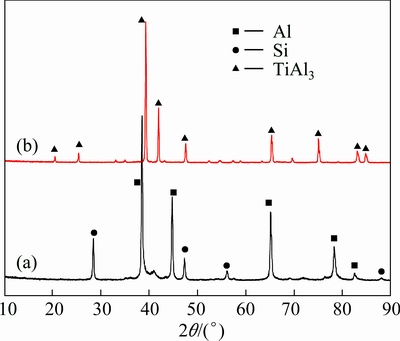
Fig. 2 XRD patterns of Al-20Si alloy coating before (a) and after (b) heat-treatment
In order to form a Ti(Al,Si)3 diffusion coating on the substrate, a heat-treatment was performed at 750 °C for 12 h. From Fig. 1(b), a new layer (80-90 μm) formed with the disappearance of the Al-20Si alloy coating. The chemical composition (68.2% Al, 22.9% Ti, 6.4% Si, 1.1% Cr, 1.4% Nb, mole fraction) and XRD pattern in Fig. 2(b) indicated that the new layer was Ti(Al,Si)3 whose Al atoms had been partly substituted by Si atoms in the ordered lattice of TiAl3. It was reported that the solubility of Si in the TiAl3 phase could reach up to 15% (mole fraction) [25]. The chemical composition of the newly formed layer in this work was in agreement with this result. Figure 3 shows the elemental maps of Al, Ti, and Si of the diffusion coating. It could be seen that the distribution of Al and Si in the diffusion coating was uniform.
3.2 Isothermal oxidation test
3.2.1 Isothermal oxidation kinetics
Figure 4 shows the isothermal oxidation kinetics curves of γ-TiAl alloy with and without the diffusion coating at 900 °C for 1000 h. It could be seen that the mass gain rate for both the bare and coated γ-TiAl alloy was fast at the initial oxidation stage (0-24 h). After that period, the mass gain rate for the coated γ-TiAl decreased greatly while the mass gain rate for the bare γ-TiAl still increased quickly. Severe spallation occurred for the bare alloy while no spallation was observed on the coated alloy. The total mass gains of the bare and coated γ-TiAl alloy after 1000 h oxidation at 900 °C were 8.6 and 2.3 mg/cm2, respectively. This result indicated that the coating decreased the oxidation rate of the γ-TiAl alloy significantly.
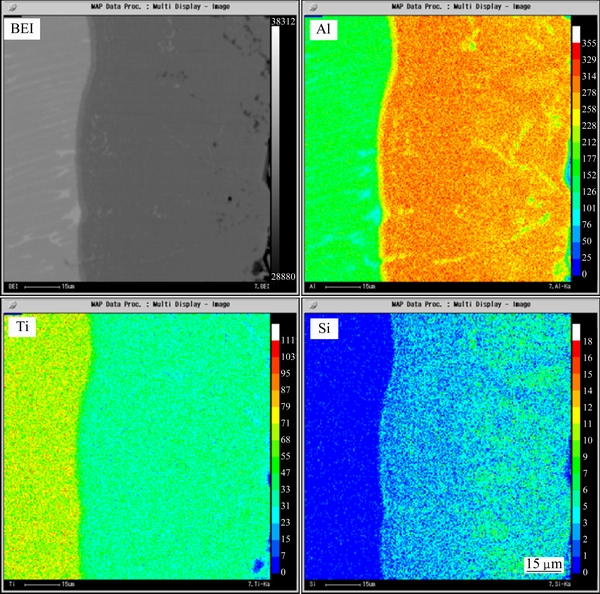
Fig. 3 EPMA elemental maps of Al, Ti, and Si of diffusion coating
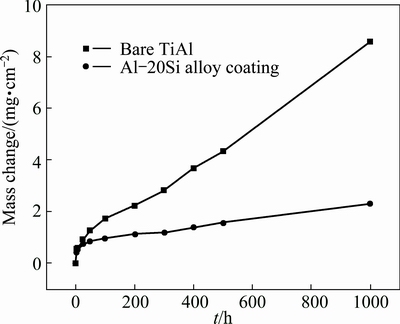
Fig. 4 Isothermal oxidation kinetics curves of γ-TiAl alloy with and without diffusion coating after oxidation at 900 °C for 1000 h
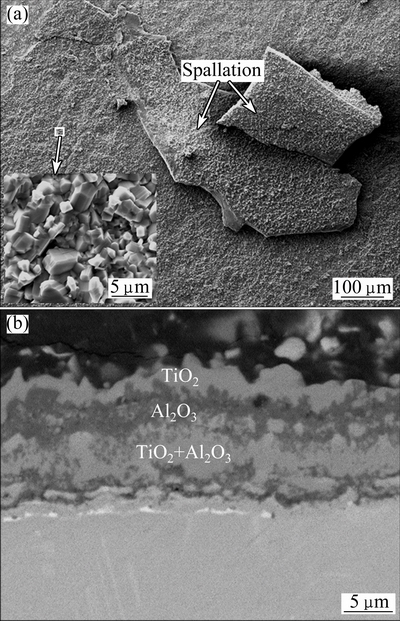
Fig. 5 SEM images of surface morphology (a) and cross-section (b) of oxidized bare γ-TiAl alloy after oxidation at 900 °C for 500 h
3.2.2 Microstructure of oxidized bare alloy
Figures 5(a) and (b) show the surface morphology, a high magnification (insert) and cross-section of the oxidized bare γ-TiAl alloy at 900 °C for 500 h, respectively. From Fig. 5(a), it could be seen that sever spallation occurred on the surface of the oxidized bare alloy. From the high magnification image (insert) in Fig. 5(a), large quantities of TiO2 crystals were observed on the surface. From Fig. 5(b), a 15 μm-thick multilayer oxide scale formed after oxidation. It consisted of an outer TiO2 layer, an intermediate Al2O3-rich layer, and an inner TiO2 and Al2O3 mixed-oxide layer, as described in Ref. [6]. This multilayer oxide scale is prone to crack and spall off. Thus, γ-TiAl alloy shows poor oxidation resistance at 900 °C in air.
3.2.3 Phase and microstructure of oxidized coating
Figure 6 shows the XRD patterns of the diffusion coating after oxidation at 900 °C for 100, 300, 500, and 1000 h, respectively. It could be seen that the main phase of the coating was Al2O3 and TiAl3 after 100 h and 300 h oxidation at 900 °C. A small amount of Ti5Si4 phase was also detected. TiO2 phase spectrum was observed until extending the oxidation time to 500 h. After 1000 h oxidation, Ti5Si3 phase could be detected. Al2O3 was still the major oxide phase formed on the coating, even though weak signals of TiO2 were detectable. This result indicated that the diffusion coating inhibited the formation of non-protective TiO2 and promoted the formation of protective Al2O3 scale.
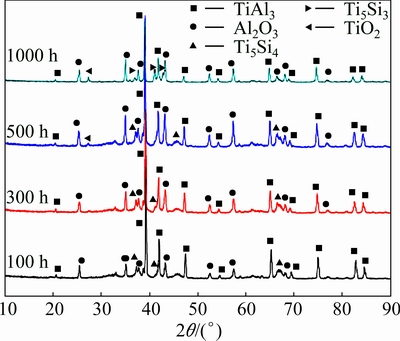
Fig. 6 XRD patterns of diffusion coating after oxidation at 900 °C for 100, 300, 500, and 1000 h, respectively
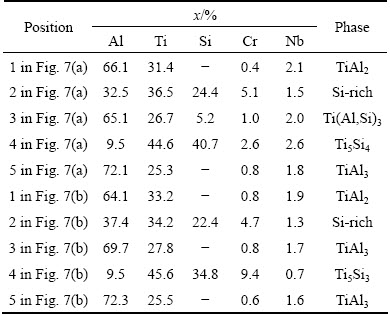
Figure 7 shows the cross-section microstructures of the diffusion coating after 300 h and 1000 h oxidation at 900 °C. Table 1 lists the EDS analysis results. Figure 8 shows the EPMA elemental maps of the diffusion coating after 300 h and 1000 h oxidation at 900 °C. From Fig. 7(a), the coating after 300 h oxidation was seen to consist of the following three distinct layers: an inner continuous light gray layer, a two-phase intermediate region identified by a dark matrix and a light second phase, an outer porous region. The 20 μm-thick inner layer (Position 1 in Fig. 7(a)) was identified as TiAl2 according to the EDS analysis. It was widely reported that high temperature exposure resulted in the formation of a TiAl2 layer due to the inward diffusion of aluminum to the substrate [6,15]. It was noticeable that this TiAl2 layer seldom contained silicon from the elemental map of Si in Fig. 8(a). This indicated that silicon barely diffused into substrate during the oxidation test. The two-phase region was found to consist of silicon-rich precipitates in an aluminum-rich matrix as shown in Fig. 8(a). The aluminum-rich matrix (Position 2 in Fig. 7(a)) was identified as Ti(Al,Si)3 according to the EDS analysis. The size of silicon-rich precipitates was about 1 μm. It contained about 25% (mole fraction) silicon according to the EDS analysis. Compared with the silicon distribution of the as-prepared coating in Fig. 3, it demonstrated that accumulation in silicon occurred in the coating, which resulted in the formation of silicon-rich precipitates. The porous region also consisted of silicon-rich precipitates in an aluminum-rich matrix. The difference was that the amount of the silicon-rich phase in the porous region was far less than that in the intermediate region while the size of the silicon-rich phase in the porous region was larger. This silicon-rich phase was identified as Ti5Si4 phase combining the EDS and XRD analysis.
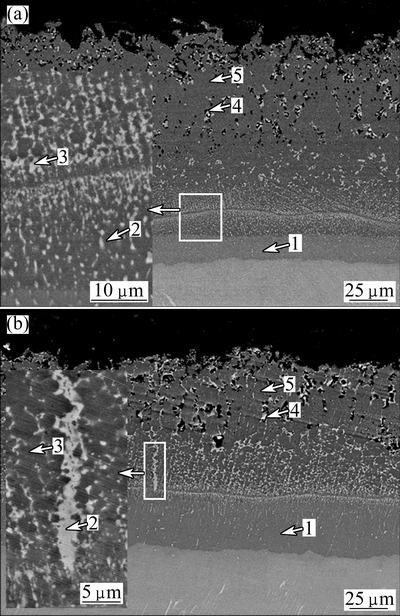
Fig. 7 SEM images of cross-section microstructures of diffusion coating after 300 h (a) and 1000 h (b) oxidation at 900 °C
Table 1 EDS analysis results of different positions in Fig. 7
From Fig. 7(b), it could be seen that the microstructure of the coating after 1000 h oxidation was similar to that after 300 h oxidation. The difference was that the thickness of the TiAl2 layer increased with the oxidation time. Furthermore, the size of the silicon-rich precipitates also increased. From the elemental map of O in Fig. 8(b), it could be seen that O had invaded into the porous region of the coating. However, no oxygen was observed in the two-phase intermediate region and the TiAl2 layer. This indicated that this multilayer coating decreased the inward oxygen diffusion effectively and thus improved the oxidation resistance of the alloy.
3.3 Cyclic oxidation test
3.3.1 Cyclic oxidation kinetics
Figure 9 shows the cyclic oxidation kinetics curves of γ-TiAl alloy with and without the diffusion coating after oxidation at 900 °C for 120 cycles. The mass gain of the bare alloy increased quickly with the oxidation time. Sever spallation was found on the surface. The total mass gain of the bare γ-TiAl reached up to 3.7 mg/cm2 after 120 cycles oxidation. While the total mass gain of the γ-TiAl with coating was 1.2 mg/cm2 after oxidation under the same test conditions for 120 cycles. Slight spallation only occurred on the edge of coated γ-TiAl sample. The results demonstrated that the coating increased the cyclic oxidation resistance of γ-TiAl alloy oxidized at 900 °C.
3.3.2 Microstructure of coating after cyclic oxidation
Figure 10 shows the surface morphologies of the diffusion coating after 1000 h oxidation and 120 cycles oxidation at 900 °C. From Fig. 10(a), it could be seen that the surface was comparatively rough. No cracks or spallation could be found after 1000 h oxidation. The surface morphology of the coating after 120 cycles oxidation was similar to that after 1000 h oxidation except some significant cracks were observed as shown in Fig. 10(b). Although cracks generated, no spallation was observed. This indicated that the oxide scale had good adhesion to the coating.
Figure 11 shows the microstructure and the tip of the crack of the diffusion coating after 120 cycles oxidation at 900 °C. It could be seen that the coating after the cyclic oxidation was also composed of three layers, which was similar to that after the isothermal oxidation. However, some significant cracks were observed in the coating after cyclic oxidation, which was in accordance with the cracks observed on the surface. The generation and propagation of these cracks were attributed to the brittleness of the TiAl3 diffusion layer compared with the TiAl substrate, combining with the thermal stress produced by the mismatch in the coefficients of thermal expansion (CTE) between the coating and substrate. After observing these cracks carefully, it could be seen that the cracks did not penetrate into the substrate and ceased to extend the bottom of the intermediate two-phase region. Moreover, a large amount of Si-rich precipitates could be found at the tip of the crack. These precipitates seemed to fill in the cracks and prevent the cracks from propagation. Although the mechanism is unclear, it indeed has a positive effect on the oxidation resistance of the coating.
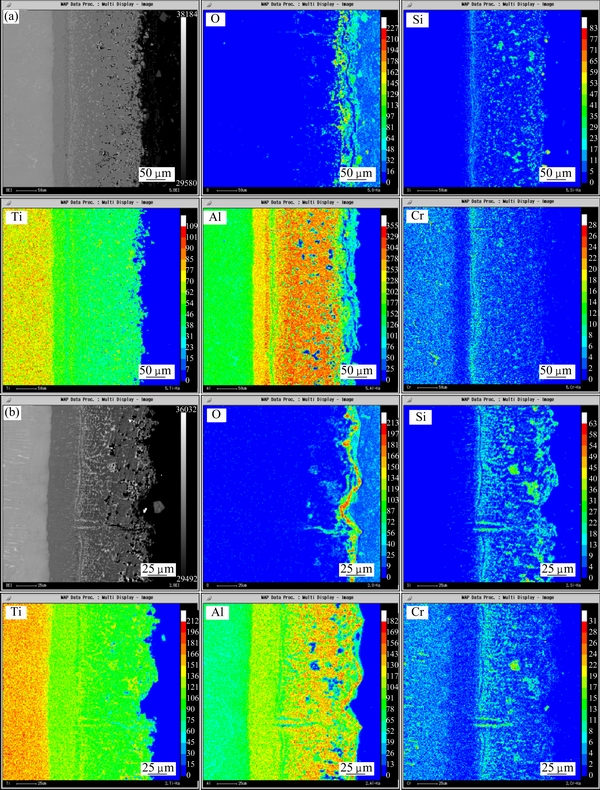
Fig. 8 EPMA elemental maps of diffusion coating after 300 h (a) and 1000 h (b) oxidation at 900 °C
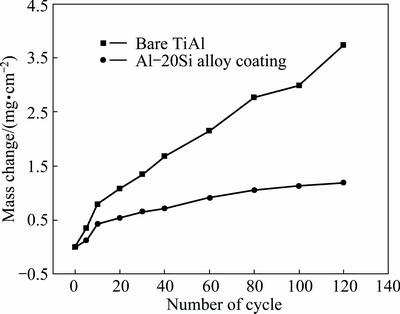
Fig. 9 Cyclic oxidation kinetics curves of γ-TiAl alloy with and without diffusion coating after oxidation at 900 °C for 120 cycles
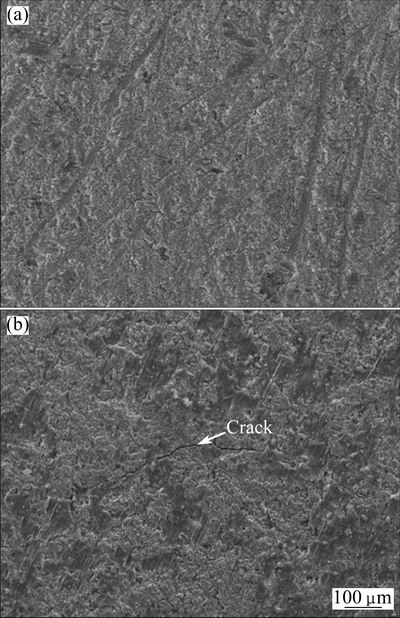
Fig. 10 SEM images of surface morphologies of diffusion coating after 1000 h oxidation (a) and 120 cycles oxidation (b) at 900 °C
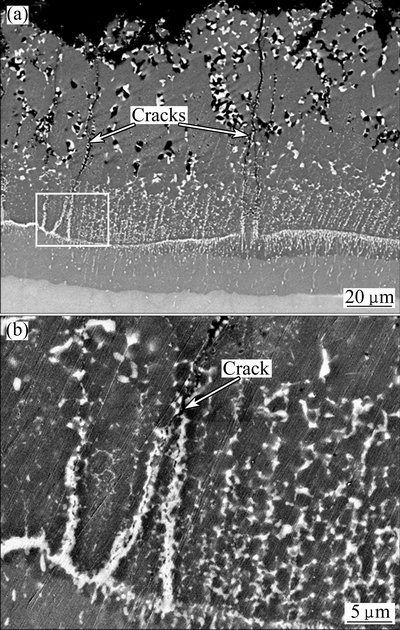
Fig. 11 SEM images of microstructure (a) and tip (b) of crack of diffusion coating after 120 cycles oxidation at 900 °C
4 Conclusions
1) A Ti(Al,Si)3 diffusion coating was prepared on the γ-TiAl alloy by cold sprayed Al-12Si coating and subsequent heat-treatment. The total mass gain of the diffusion coating after 1000 h oxidation was 2.3 mg/cm2, only a quarter of that of the bare alloy. This was mainly because the diffusion coating promoted the formation of Al2O3 scale and thus decreased the inward oxygen diffusion.
2) After 1000 h oxidation, the diffusion coating was degraded into three layers: an inner TiAl2 layer with a thickness of about 70 μm, a two-phase intermediate layer composed of a Ti(Al,Si)3 matrix and Si-rich precipitates with a thickness of about 50 μm, a porous layer with a thickness of about 50 μm. The degradation of the coating mainly resulted from the inter-diffusion between the coating and substrate.
3) Cracks induced by thermal stress were observed in the coating after cyclic oxidation. However, they stopped propagation at the bottom of the intermediate layer. It might be attributed to the filling of the Si-rich precipitates.
References
[1] APPEL F, BROSSMANN U, CHRISTOPH U, EGGERT S, JANSCHEK P, LORENZ U. Recent progress in the development of gamma titanium aluminide alloys [J]. Advanced Engineering Materials, 2000, 2(11): 699-720.
[2] CLEMENS H, KESTLER H. Processing and applications of intermetallic gamma-TiAl-based alloys [J]. Advanced Engineering Materials, 2000, 2(9): 551-570.
[3] KOTHARI K, RADHAKRISHNAN R, WERELEY N M. Advances in gamma titanium aluminides and their manufacturing techniques [J]. Progress in Aerospace Sciences, 2012, 55: 1-16.
[4] MCKEE D, HUANG S. The oxidation behavior of gamma-titanium aluminide alloys under thermal cycling conditions [J]. Corrosion Science, 1992, 33(12): 1899-1914.
[5] SCHMIEDGEN M, GRAAT P, BARETZKY B, MITTEMEIJER E. The initial stages of oxidation of γ-TiAl: An X-ray photoelectron study [J]. Thin Solid Films, 2002, 415(1): 114-122.
[6] GAUTHIER V, DETTENWANGER F, SCHUTZE M, SHEMET V, QUADAKKERS W. Oxidation-resistant aluminide coatings on γ-TiAl [J]. Oxidation of Metals, 2003, 59(34): 233-255.
[7] XU Y, MIAO Q, LIANG W P, YANG J J, YAO Z J. Interdiffusion performance and oxidation behaviours of NiCrAlY/Al coatings on gamma-TiAl [J]. Surface Engineering, 2014, 30(1): 64-70.
[8] GAO J, HE Y, GAO W. Electro-codeposition of Al2O3-Y2O3 composite thin film coatings and their high-temperature oxidation resistance on γ-TiAl alloy [J]. Thin Solid Films, 2012, 520(6): 2060-2065.
[9] GAUTHIER V, DETTENWANGER F, SCHUTZE M. Oxidation behavior of γ-TiAl coated with zirconia thermal barriers [J]. Intermetallics, 2002, 10(7): 667-674.
[10] BRAUN R, FROHLICH M, BRAUE W, LEYENS C. Oxidation behaviour of gamma titanium aluminides with EB-PVD thermal barrier coatings exposed to air at 900 °C [J]. Surface and Coatings Technology, 2007, 202(4): 676-680.
[11] IZUMI T, NISHIMOTO T, NARITA T. Superior long-term oxidation resistance of Ni-Al coated TiAl alloys [J]. Intermetallics, 2005, 13(7): 727-732.
[12] XIANG Z D, ROSE S R, DATTA P K. Conditions for formation of coherent aluminide coatings on gamma-TiAl by pack cementation process [J]. Surface Engineering, 2002, 18(5): 373-380.
[13] SASAKI T, YAGI T, WATANABLE T, YANAGISAWA A. Aluminizing of TiAl-based alloy using thermal spray coating [J]. Surface and Coatings Technology, 2011, 205(13,14): 3900-3904.
[14] VARLESE F A, TULUI M, SABBADINI S, PELLISSERO F, SEBASTIANI M, BEMPORAD E. Optimized coating procedure for the protection of TiAl intermetallic alloy against high temperature oxidation [J]. Intermetallics, 2013, 37: 76-82.
[15] MIYAKE M, TAJIKARA S, HIRATO T. Fabrication of TiAl3 coating on TiAl-based alloy by Al electrodeposition from dimethylsulfone bath and subsequent annealing [J]. Surface and Coatings Technology, 2011, 205(21-22): 5141-5146.
[16] WANG J Q, KONG L Y, LI T F, XIONG T Y. Oxidation behavior of thermal barrier coatings with a TiAl3 bond coat on gamma-TiAl alloy [J]. Journal of Thermal Spray Technology, 2015, 24(3): 467-475.
[17] XIONG H P, MAO W, XIE Y H, MA W L, CHEN Y F, LI X H. Liquid-phase siliconizing by Al-Si alloys at the surface of a TiAl-based alloy and improvement in oxidation resistance [J]. Acta Materialia, 2004, 52(9): 2605-2620.
[18] XIONG H P, MAO W, XIE Y H, CHENG Y Y, LI X H. Formation of silicide coatings on the surface of a TiAl-based alloy and improvement in oxidation resistance [J]. Materials Science and Engineering A, 2005, 391(1-2): 10-18.
[19] SWADZBA L, MOSKAL G, HETMANCZYK M, MENDALA B, JARCZYK G. Long-term cyclic oxidation of Al-Si diffusion coatings deposited by arc-PVD on TiAlCrNb alloy [J]. Surface and Coatings Technology, 2004, 184(1): 93-101.
[20] XIANG Z, ROSE S, DATTA P. Codeposition of Al and Si to form oxidation-resistant coatings on γ-TiAl by the pack cementation process [J]. Materials Chemistry and Physics, 2003, 80(2): 482-489.
[21] GORAL M, MOSKAL G, SWADZBA L. The influence of Si on structure of aluminide coatings deposited on TiAl alloy [J]. Journal of Achievements in Materials and Manufacturing Engineering, 2006, 18(1-2): 463-466.
[22] GORAL M, MOSKAL G, SWADZBA L. The influence of Si on oxidation resistance of aluminide coatings on TiAl alloy [J]. Journal of Achievements in Materials and Manufacturing Engineering, 2006, 18(1-2): 459-462.
[23] LI Yong-quan, XIE Fa-qin, WU Xiang-qiang. Microstructure and high temperature oxidation resistance of Si-Y co-deposition coatings prepared on TiAl alloy by pack cementation process [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(3): 803-810.
[24] XIONG H P, SHEN Q, LI J G, ZHANG L M, YUAN R Z. Design and microstructures of Ti/TiAl/Al system functionally graded material [J]. Journal of Materials Science Letters, 2000, 19: 989-993.
[25] CAROLAN D, TUKOVIC Z, MURPHY N, IVANKOVIC A. Arbitrary crack propagation in multi-phase materials using the finite volume method [J]. Computational Materials Science, 2013, 69: 153-159.
冷喷涂制备γ-TiAl表面Ti(Al,Si)3扩散涂层的高温氧化行为
王吉强,孔令艳,李铁藩,熊天英
中国科学院 金属研究所,沈阳 110016
摘 要:通过冷喷涂Al-20Si合金涂层及后续热扩散处理在γ-TiAl合金表面制备Ti(Al,Si)3扩散涂层。对涂层进行900 °C下1000 h 等温氧化和120次循环氧化来测试其抗氧化性能。通过扫描电子显微镜、X-射线衍射以及电子探针分析涂层氧化前后的组织和相转变。结果表明:该扩散涂层具有良好的抗氧化能力,其氧化增重只有基体合金的四分之一。由于在氧化过程中涂层与基体发生互扩散,氧化后的涂层退化为3层:TiAl2内层、由Ti(Al,Si)3和富Si相组成的中间层以及多孔层。
关键词:Ti(Al,Si)3扩散涂层;γ-TiAl合金;冷喷涂;热处理;高温氧化
(Edited by Xiang-qun LI)
Foundation item: Project (50971127) supported by the National Natural Science Foundation of China
Corresponding author: Ling-yan KONG, Tel: +86+24-23971719; E-mail: lykong@imr.ac.cn
DOI: 10.1016/S1003-6326(16)64214-0
Abstract: A Ti(Al,Si)3 diffusion coating was prepared on γ-TiAl alloy by cold sprayed Al-20Si alloy coating, followed by a heat-treatment. The isothermal and cyclic oxidation tests were conducted at 900 °C for 1000 h and 120 cycles to check the oxidation resistance of the coating. The microstructure and phase transformation of the coating before and after the oxidation were studied by SEM, XRD and EPMA. The results indicate that the diffusion coating shows good oxidation resistance. The mass gain of the diffusion coating is only a quarter of that of bare alloy. After oxidation, the diffusion coating is degraded into three layers: an inner TiAl2 layer, a two-phase intermediate layer composed of a Ti(Al,Si)3 matrix and Si-rich precipitates, and a porous layer because of the inter-diffusion between the coating and substrate.


