
Synthesis of crystalline γ-Al2O3 with high purity
YI Jian-hong(仪建红)1, SUN You-yi(孙友谊)1, GAO Jian-feng(高建峰)2, XU Chun-yan(徐春彦)2
1. College of Materials Science and Engineering, North University of China, Taiyuan 030051, China;
2. Department of Chemistry, North University of China, Taiyuan 030051, China
Received 13 July 2008; accepted 20 January 2009
Abstract:
Crystalline γ-AlO(OH) was synthesized by the precipitation of sodium aluminate and oxalic acids in aqueous solution. And then γ-AlO(OH) was successfully transferred to γ-Al2O3 after subsequent high temperature heat treatment. The effects of reaction conditions on formation of γ-AlO(OH) and γ-Al2O3 were further investigated in detail. The XRD analysis shows that the complete formation of crystalline γ-Al2O3 is at pH 8-9, reaction temperature of 93-96 ℃ and calcination temperature of higher than 400 ℃. The product of γ-Al2O3 contains impurity, including iron, calcium and silicon ion with a low content of about 0.01% and has large specific surface area and high pore volume of 269.9 m2/g and 0.57 mL/g, which can be applied in catalysts and catalyst supports.
Key words:
γ-Al2O3; high purity; sodium aluminate; oxalic acids;
1 Introduction
The preparation of alumina powders has been an interesting field due to their applications in many areas of modern industry such as electronics, metallurgy, optoelectronics, catalysts and fine ceramic composites [1-3]. In recent years, because they can be used as adsorbents, filters, catalysts and catalyst supports, especial attention has been focused on the preparation of γ-Al2O3 powders with high purity by various routes such as precipitation, gas phase deposition, sol-gel and hydrothermal methods[4-8]. Among these methods, precipitation is the most commonly used method not only because it can produce high quality powders but also it is cheap[9].
The industrial production of alumina is typically via the calcination of the precipitates of aluminum nitrate or aluminum chloride and antalkali[10-11]. This process consists of bauxite ore digestion, liquor clarification, gibbsite crystallization and calcinations of Al(Ⅲ)- containing hydroxides. In addition, highly crystallized γ-Al2O3 nanoparticle was synthesized hydrothermally in supercritical water by using a continuous flow reaction system from the starting reagent of Al(NO3)3·9H2O[12]. The effects of temperature, pressure and reaction time on the formation of γ-Al2O3 were studied under supercritical conditions of water. However, these processes involve complicated product line that requires anticorrosion of equipment and low yields. Recently, there are some repots about synthesis of γ-Al2O3 with high purity by hydrolysis of aluminium isopropylate[13]. It is well known that the aluminum with high purity is required to be γ-Al2O3 by the method. So, the process does not only involve complicated product line, but also high cost. To solve these problems, structure and precipitation mechanism of sodium aluminate(SA) solution have been widely investigated to obtain γ-AlOOH (or γ-Al2O3)[14-16]. But, this is a main bottleneck that γ-Al2O3 powder with high purity is difficult to be obtained by these methods directly from SA solution.
So, the γ-Al2O3 with high purity was synthesized by a new process, in which the sodium aluminate solution was treated by the HG solid desiliconization agents, and then the precipitates were formed in the presence of oxalic acid solution.
2 Experimental
2.1 Materials
Sodium aluminate was purchased from Shangxi Alumina Factory, China; carbon dioxide was supplied by Taiyuan Iron and Steel Co. Ltd., oxalic acid was purchased from Chemical Plant of Shanxi, China; and HG solid desiliconization agents, solid desodium agentⅠand solid desodium agent Ⅱ were prepared.
2.2 Purification of sodium aluminate
Sodium aluminate was purified by the HG solid desiliconization agents as follows. The calces and HG solid desiliconization agents were dissolved in sodium aluminate under vigorous stirring at 50 ℃. In addition, other calces were further added to the mixing solution, which was repeated two times. The mixing solution was left for 2 h with rapid stirring for azeotropic distillation. After then, it was cooled to room temperature and the precipitates were removed. And pH of the filtrate was adjusted to 13 by injecting with CO2. At last, the oxalic acid was added into the filtrate, resulting in the formation of the precipitates that was again removed. The purification procedure was repeated two times.
2.3 Synthesis of γ-Al2O3
The preparation of γ-Al2O3 was typically via the calcination of the precipitation of sodium aluminate and oxalic acid in aqueous solution. In the typical experiment, oxalic acid and NaAlO2 were pumped together at a rate of 180 mL/min into aqueous solution at 25 ℃. And then, the mixing solution was left for 2.5 h under vigorous stirring at 100 ℃ and the white precipitate was formed. The white precipitate was aged, separated and washed for two times with distilled water until the filtrate became neutral, and then was dispersed in aqueous solution containing solid desodium agentⅠunder vigorous stirring to form a highly uniform mixture, which was subsequently transferred into a conical flask for azeotropic distillation. After distilling at 100 ℃ (the boiling point of water) for 1 h and cooling to room temperature, the precipitate was separated and washed with distilled water. The purification procedure was repeated in the presence of solid desodium agent Ⅱ. The precipitate was recovered by centrifugation and dried in an vacuum drying oven at 150 ℃ for 12 h. Calcination was further carried out at 300, 350, 400, 450 and 500 ℃ for 4 h with the temperature increasing at 2 ℃/min.
2.4 Characterization
Inductively coupled argon plasma analysis(ICP) was used to determine the concentration of iron, alumina, calcium and silicon. The instrument used was a Thermo Jarrell Ash Atom Scan 25 spectometer. Solutions containing the sample to be assessed were prepared by digesting the powder with concentrated acid such as HCl under reflux for periods of 2-7 h. Since it is a comparative technique, the relationship between emission intensity and concentration was determined using blank solutions and standard materials whose concentrations were known.
XRD patterns were recorded with a Bruker D8 Advance X-ray diffractometer with Cu Kα irradiation (40 kV, 40 mA). The average crystal size was estimated by applying the Scherrer equation to the apparent full width at half maximum intensity(FWHM) of (440) peak of γ-Al2O3, with silicon as a standard of the instrumental line broadening.
The size and morphology of the particles were observed at room temperature on a scanning electron microscope (SEM, Model JSM-6700, JEOL, Tokyo, Japan).
Nitrogen adsorption and desorption isotherms were performed in a Micromeritics ASAP 2000 volumetric adsorption system.
3 Results and discussion
3.1 Synthesis of γ-Al2O3 with high purity
γ-Al2O3 with high purity was synthesized by the direct precipitation of SA solution and oxalic acid. This is a strongly interrelated system that may be established between the base-catalyzed decomposition reaction and the ionization process of oxalic acid when oxalic acid solution is dropped into NaAlO2 solution. pH of NaAlO2 solution can be finely tuned and retained in a favorable range for polymerization of ![]() into indissoluble γ-AlO(OH) with porous structure, namely,
into indissoluble γ-AlO(OH) with porous structure, namely,
NaAlO2+C2H2O4→![]() +(NaCOO)2 (1)
+(NaCOO)2 (1)
![]() →γ-AlO(OH)?nH2O+(1-n)H2O (2)
→γ-AlO(OH)?nH2O+(1-n)H2O (2)
And then γ-AlO(OH) was further heat treated at 450? and transferred to γ-Al2O3. The formation of nano-sized γ-Al2O3 with high purity is proved by Fig.1 and Table 1. Fig.1(a) shows the typical diffraction peaks (111), (311), (400) and (440) of γ-Al2O3 with a cubic structure[15] (unit cell parameters are a=0.790 nm, b=0.790 nm, and c=0.790 nm) (JCPDS Card No. 10-0425), indicating the formation of crystalline γ-Al2O3. All the diffraction peaks exhibit a high degree of broadness due to the fine nature and a less degeneracy in the crystallites. Based on the Debye–Scherrer equation[17], the mean crystallite size of γ-Al2O3 calculated from the full width at half-maximum of the isolated (311), (400) and (440) diffraction peaks are 10.9, 13.9 and 18.8 nm, respectively. The size and morphology of the particles were further characterized by the SEM image as shown in Fig.1(b). The combined effect of liquid- attached Al(OH)3 and nano-sized γ-Al2O3 seeds on reducing γ-Al2O3 particle size is remarkable. γ-Al2O3 particles obtained with liquid-attached Al(OH)3 and nano-sized γ-Al2O3 as seeds have an average size of 40.0-50.0 nm and show less agglomeration.
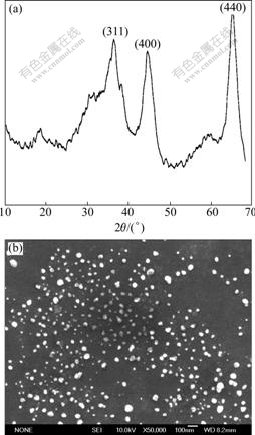
Fig.1 XRD pattern (a) and SEM image (b) of γ-Al2O3 powders synthesized by heating precipitation of sodium aluminate and oxalic acids at 450 ℃
Table 1 Content of impurity in γ-Al2O3 prepared and its physical properties
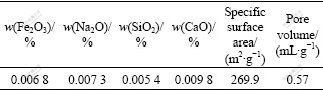
The purity of γ-Al2O3 prepared by the present method was further determined by the inductively coupled argon plasma analysis as shown in Table 1. The result shows that the content of impurity (eg. iron, calcium or silicon ion) is lower than 0.1%, which is very important to obtain γ-Al2O3 with high catalyst property. Furthermore, the large specific surface area and high pore volume (269.9 m2/g and 0.57 mL/g) are also observed in Table 1, which are also very important to γ-Al2O3 with high catalyst property.
3.2 Effects of preparing conditions on purity and formation of γ-Al2O3
3.2.1 Purification of sodium aluminate
During the preparation of high purity γ-Al2O3 from sodium aluminate solution in the present process, the removal of silicate and iron ions in the sodium aluminate solution has a considerable effect on the grade of γ-Al2O3 product. The mass ratio of Al2O3 to SiO2 or Fe2O3 (A/S) in the sodium aluminate solution can rise up to 3 000 by desiliconization in the previous process [18-19]. However, it must be raised to 10 000 in order to obtain highly qualified product in the sintering process. So, the sodium aluminates were purified by the two-stage desiliconization procedure and a novel desiliconization agent was developed to remove silicate, calcium and iron ions completely.
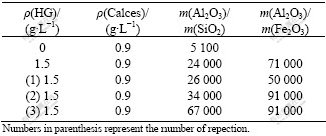
In the first stage, the novel HG solid desiliconization agents and Ca(OH)2 were usually used to remove silicate and iron ions completely as shown in Table 2. The result shows that the mass ratios of Al2O3 to SiO2 and Al2O3 to Fe2O3 in the sodium aluminate solution are raised to 24 000 and 71 000, respectively, indicating that the HG solid desiliconization agents have higher desiliconization capacity and efficiency compared with other desiliconization methods[20]. Generally, when the desiliconization agents are used to remove silicate and iron ions from sodium aluminate solution, the loss of aluminum ion is about 20%. Here, when the novel HG solid desiliconization agents are used, the loss is reduced to be about 10%. Moreover, the HG solid desiliconization agents can be repeatedly used for at least three times according to the A/S of 67 000 or 91 000.
Table 2 Effect of desilication on removing silicate ions and iron ions from sodium aluminate
Secondly, according to above desiliconization procedure, Ca(OH)2 was introduced to the sodium aluminates, which is usually used to remove silicate and iron ions completely. The decalcium is also necessary in order to obtain qualified product in the sintering process. The calcium ion can be effectively removed by the oxalic acid as shown in Table 3. The result shows that the mass ratio of Al2O3 to CaO (A/S) in the sodium aluminate solution is raised to 14 2000, suggesting that the oxalic acid is a good agent of removing calcium ions.
Table 3 Effect of decalcium on removing Ca ions from sodium aluminate
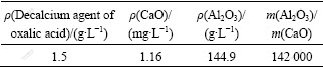
3.2.2 pH of reaction solution
While preparing high purity γ-Al2O3 by the present method, not only the purification of sodium aluminate is important, but also the solution pH is important for the synthesis process. XRD patterns of as-dried samples obtained in various pH solutions by the oxalic acid route are shown in Fig.2. When pH values of the solution are lower than 8.5(4.0, 6.0 and 7.5), non-crystalline AlO(OH) forms and the crystallinity of AlO(OH) increases with the increase in pH. Contrarily, when the solution pH values are higher than 8.5(9.5, 10.5 and 12.0), the typical diffraction patterns (020), (120), (031), (051) of γ-AlO(OH) with a cubic structure[21] and (002), (110) of β-Al(OH)3[22] are observed, suggesting the formation of the Al(OH)3 and γ-AlO(OH), in which the Al(OH)3 is easy to transfer to Al2O3·nH2O as shown in following reaction:
Al(OH)3→Al2O3·nH2O+H2O (3)
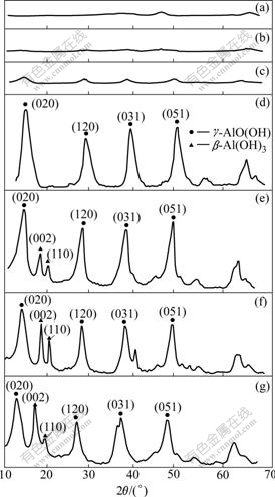
Fig.2 XRD patterns of product synthesized in various solution pH: (a) pH=4.0; (b) pH=6.0; (c) pH=7.5; (d) pH=8.5; (e) pH= 9.5; (f) pH=10.5; (g) pH=12.0
So, pH of reaction solution is chosen to be 8-9 to obtain γ-AlO(OH) with high purity (pH=8.5), which is very key to obtain γ-Al2O3 with high purity after calcinations[14].
3.2.3 Reaction temperature
Effects of reaction temperature on formation and purity of γ-Al2O3 were further examined as shown in Fig.3. When the reaction temperature is 30-90 ℃, the sample is non-crystalline AlO(OH). The higher temperature (93 ℃) leads to the continual formation of γ-AlO(OH) with high purity according to the typical diffraction patterns (020), (120), (031) and (051) of γ-AlO(OH) with a cubic structure. However, when the temperature is further increased to 96 ℃ or 98℃, the β-Al(OH)3 is formed according to the appearance of the typical diffraction patterns of (002) and (110), which is easy to transfer to α-Al2O3 after the calcination. It is well known that the formation of AlO(OH) is kinetically favored and that the formation of Al(OH)3 is thermodynamically favored. From a standpoint of kinetics, AlO(OH) is easier to form by the precipitation of sodium aluminate and oxalic acids at low temperature. However, this sodium aluminate has a tendency to hydrolyze to β-Al(OH)3 at high temperature. So, the range of 93-96 ℃ is chosen to obtain γ-Al2O3 with high purity.
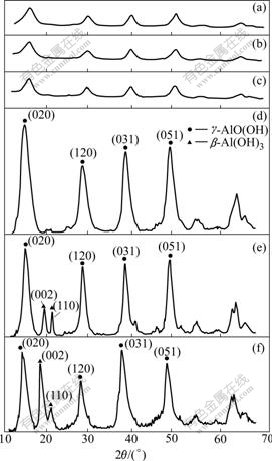
Fig.3 XRD patterns of product synthesized at various reaction temperatures: (a) 30 ℃; (b) 60 ℃; (c) 90 ℃; (d) 93 ℃; (e) 96 ℃; (f) 98 ℃
3.2.4 Concentration of oxalic acid and sodium aluminate
During the preparation of γ-Al2O3 from calcination of crystalline γ-AlO(OH) in the present process, the removal of sodium ions in γ-AlO(OH) is also very important for obtaining γ-Al2O3 product with high purity. The concentrations of sodium ions in γ-AlO(OH) were determined by concentration of oxalic acid and sodium aluminate as shown in Table 4. The result shows that the concentration of sodium ions increases with increase in concentration of NaAlO2. Moreover, it is more difficult to remove the sodium ions from γ-AlO(OH) for higher concentration than 80 g/L, resulted from γ-AlO(OH) encapsulating sodium ions. At the same time, the concentration of sodium ions also increases with increase in concentration of oxalic acid because the Al2O3·nH2O is formed at higher concentration than 55 g/L as discussion above, which is also easy to adsorb the sodium ion. What’s more, the sodium ion is difficult to be removed by washing with water from Al2O3·nH2O. So, here the concentration of oxalic acid and NaAlO2 are controlled to be 45-50 g/L and 75-80 g/L in order to obtain γ-Al2O3 with high purity. As can be seen from the Table 4, the sodium ion is effectively removed by the desodium agent. Especially, after γ-AlO(OH) was purified by the desodium agentⅠand desodium agent Ⅱ, the sodium ions have been almost removed completely. The mechanism of desodium reaction can be represented as follows. NH4+ of desodium agent can displace sodium ions adsorbed in γ-AlO(OH), and then sodium ions are easy to be washed with the hot water. The different desodium agent can displace sodium ions adsorbed in various parts of γ-AlO(OH).
Table 4 Effect of oxalic acid and NaAlO2 concentration on removing Na ions from AlOOH
3.2.5 pH of washing water
During the washing γ-AlO(OH) with aqueous solution, pH of washing water is also very important for obtaining γ-Al2O3 product with high purity as shown in Fig.4. The result shows the typical diffraction patterns of β-Al(OH)3 and γ-AlO(OH) at pH of 4.0 and 7.0, and the typical diffraction patterns of pure γ-AlO(OH) at pH 9.0. This suggests that γ-AlO(OH) washed with water at pH<9.0 is easy to transfer to β-Al(OH)3. The reaction can be expressed as follows:
γ-AlO(OH)+H2O→β-Al(OH)3 (4)
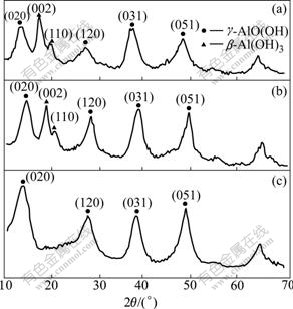
Fig.4 XRD patterns of products affect washing γ-AlO(OH) powder with water at various pH: (a) pH=4.0; (b) pH=7.0; (c) pH=9.0
3.2.6 Calcined temperature
XRD patterns of as-dried samples obtained by the oxalic acid route and their calcined products are shown in Fig.5. From Fig.5, XRD patterns for γ-AlO(OH) andγ-Al2O3 can be clearly seen in at the calcined temperature of 300 ℃ and 350 ℃.
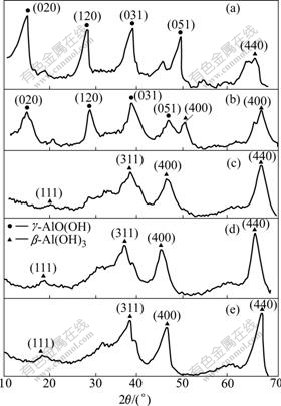
Fig.5 XRD patterns of products after calcining γ-AlO(OH) powders at various temperatures: (a) 300 ℃; (b) 350 ℃; (c) 400 ℃; (d) 450 ℃; (e) 500 ℃
The calcined products at the temperatures of 400, 450 and 500 ℃ show diffraction patterns matching with the standard diffraction data for γ-Al2O3 as shown in Fig.5[16], indicating that γ-AlO(OH) is effectively transferred to γ-Al2O3 at the higher calcined temperature than 400 ℃ as follows:
2γ-AlO(OH)·nH2O→γ-Al2O3+2(1-n)H2O (5)
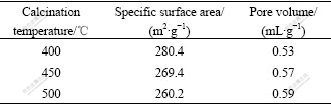
The specific surface area and pore volume of γ-Al2O3 prepared in various calcined temperatures were further investigated as shown in Table 5. The result shows that the specific surface area decreases and the pore volume increases with the increase in calcined temperatures. The large specific surface area and high pore volume of γ-Al2O3 are all necessary for γ-Al2O3 with high catalyst property. So, the calcined temperature of product is chosen to be 450 ℃.
Table 5 Properties of γ-Al2O3 powders calcined at various calcined temperatures
4 Conclusions
1) γ-Al2O3 with high purity was prepared in the present work. The effect of reaction conditions on the formation of γ-Al2O3 with high purity was investigated in detail.
2) The favorable ranges of pH and reaction temperature are 8-9 and 93-96 ℃, respectively. In addition, the concentrations of oxalic acid and sodium aluminate are controlled to be 45-50 g/L and 75-80 g/L, respectively. When the temperature of calcination is higher than 400 ℃, the AlO(OH) is changed completely to γ-Al2O3.
References
[1] SHOJAIE-BAHAABAD M, TAHERI-NASSAJ E. Economical synthesis of nano alumina powder using an aqueous sol–gel method [J]. Materials Letters, 2008,62(19): 3364-3366.
[2] FRANKLIN C, DANIEL A, OSVALDO F G. Dehydrogenation of propane on chromia/alumina catalysts promoted by tin [J]. Catalysis Today,2008, 133/135: 800-804.
[3] HUANG Cheng-liang, WANG Jun-jie, YEN Fu-su, HUANG Chi-yuen. Microwave dielectric properties and sintering behavior of nano-scaled (α+θ)-Al2O3 ceramics [J]. Materials Research Bulletin, 2008,43(6): 1463-1471.
[4] HICHAM Z, DANIEL B, OUAFAE A, TARIK C. A comparative study of the adsorption and desorption of o-xylene onto bentonite clay and alumina [J]. Journal of Hazardous Materials, 2008,153(1/2): 852-859.
[5] BOLT H, KOCH F, RODET J L, KARPOV D, MENZEL S. Al2O3 coatings deposited by filtered vacuum arc—Characterization of high temperature properties [J]. Surface and Coatings Technology,1999, 116/119: 956-962.
[6] EMRAH O, DARREL H, J?NOS S. NOx reduction on a transition metal-free γ-Al2O3 catalyst using dimethylether (DME) [J]. Catalysis Today,2008, 136(1/2): 46-54.
[7] WU J C S, LIN Shang-jie. Novel BN supported bi-metal catalyst for oxydehydrogenation of propane [J]. Chemical Engineering Journal,2008, 140(1/3): 391-397.
[8] ESWARAMOORTHI I, SUNDARAMURTHY V, NIKHIL D, DALAI A K, ADJAYE J. Application of multi-walled carbon nanotubes as efficient support to NiMo hydrotreating catalyst [J]. Applied Catalysis A: General,2008, 339(2): 187-195.
[9] POTDAR H S, JUN K W, JONG W B, KIM S M, LEE Y J. Synthesis of nano-sized porous γ-alumina powder via a precipitation/digestion route [J]. Applied Catalysis A: General, 2007,321(2): 109-116.
[10] SCHMIDT F. New catalyst preparation technologies—observed from an industrial viewpoint [J]. Applied Catalysis A: General, 2001, 221(1/2): 15-21.
[11] ISMAGILOV Z R, SHKRABINA R A, KORYABKINA N A. New technology for production of spherical alumina supports for fluidized bed combustion [J]. Catalysis Today,1999, 47(1/4): 51-71.
[12] TAKIO N, KEITARO M, NAZRUL M I, YUKIYA H, HIROMICHI H. Rapid synthesis of γ-Al2O3 nanoparticles in supercritical water by continuous hydrothermal flow reaction system [J]. J Supercritical Fluids, 2008, 46(2): 129-136.
[13] ZHANG Mei-ge, LIN Lan-sheng, ZHAO Gao-lun, BAI Dong-wen. Ultrafine high-purity alumina prepared by hydrolysis of aluminium isopropylate [J]. Chinese Journal of Applied Chemistry, 1993, 10(5): 99-101. (in Chinese)
[14] CAI Wei-quan, LI Hui-quan, ZHANG Yi. Influences of processing techniques of the H2O2-precipitated pseudoboehmite on the structural and textural properties of γ-Al2O3 [J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects,2007, 295(1/3): 185-192.
[15] CAI Wei-quan, LI Hui-quan, ZHANG Yi. Azeotropic distillation-assisted preparation of macro-mesostructured γ-Al2O3 nanofibres of crumpled sheet-like morphology [J]. Materials Chemistry and Physics,2006, 96(1): 136-139.
[16] BARAKAT M A, EL-SHEIKH S M, FARGHLY F E. Regeneration of spent alkali from aluminum washing [J]. Separation and Purification Technology, 2005, 46: 214-218.
[17] CHANDA S C, MANNA A, VIJAYAN V, PRANABA K N, ASHOK M, ACHARYA H N. PIXE & XRD analysis of nanocrystals of Fe, Ni and Fe2O3 [J]. Materials Letters,2007, 61(28): 5059-5062.
[18] YUAN Jiong-liang, ZHANG Yi. Desiliconization reaction in sodium aluminate solution by adding tricalcium hydroaluminate [J]. Hydrometallurgy,2009, 95(1/2): 166-169.
[19] NOWORYTA A. Mathematical model of the desiliconization of aluminate solutions [J]. Hydrometallurgy,1981, 7(1/2): 107-115.
[20] TOSHIAKI O, KATSUNORI F, SHIGEAKI M. Development of zirconia electrolyte sensor with auxiliary electrode for the in situ measurement of dissolved silicon in molten iron [J]. Sensors and Actuators B: Chemical,1990, 1(1/6): 203-209.
[21] CHEN Xiang-ying. ZHANG Zhong-jie, LI Xue-liang, LEE S W. Controlled hydrothermal synthesis of colloidal boehmite (γ-AlOOH) nanorods and nanoflakes and their conversion into γ-Al2O3 nanocrystals [J]. Solid State Communications, 2008, 145: 368-373.
[22] TANG Yue-feng, LI Ai-dong, LU Yi-nong, LI Xiao-yun, SHI Shu-zhe, LING Zhi-da. Preparation of core/shell structure of α-Al(OH)3-SiO2 by heterogeneous nucleation-and-growth processing [J]. Journal of Sol-Gel Science and Technology, 2003, 27(3): 263-265.
Foundation item: Project(N 20031012) supported by Youth Science Foundation of Shanxi Province, China; Project(20080320ZX) supported by the University’s Science and Technology Exploiture of Shanxi Province, China
Corresponding author: GAO Jian-feng; Tel: +86-351-3628189, E-mail: jianfenggao@163.com
DOI: 10.1016/S1003-6326(08)60435-5


