Trans. Nonferrous Met. Soc. China 25(2015) 3904-3908
Reactive diffusion in Mg-Gd binary system at 773 K
Wei-wen ZHENG, Xing-gang LI, Kui ZHANG, Yong-jun LI, Ming-long MA, Guo-liang SHI
State Key Laboratory for Fabrication and Processing of Nonferrous Metals, General Research Institute for Nonferrous Metals, Beijing 100088, China
Received 7 February 2015; accepted 14 June 2015
Abstract:
The reactive diffusion in Mg-Gd binary system was studied at 773 K by optical microscopy (OM), scanning electron microscopy (SEM) and electron probe micro-analysis (EPMA). After annealing at 773 K for 12-48 h, four different intermetallic layers, Mg5Gd, Mg3Gd, Mg2Gd and MgGd, form at the Mg/Gd interfaces in the diffusion couples. The thicknesses of intermetallic layers δi (i stands for the phases of Mg5Gd, Mg3Gd, Mg2Gd and MgGd, respectively) are proportional to the square root of annealing time t1/2, which indicates that the growth behavior of the intermetallics is controlled by the diffusion rate. The ratio of thickness of each intermetallic layer to the total thickness is constant with increasing the annealing time, which means that the growth behavior is constant at a certain annealing temperature. The diffusion coefficient of Gd in different intermetallics was calculated by Matano method.
Key words:
Mg-Gd; reactive diffusion; annealing; intermetallics;
1 Introduction
As the lightest structural metallic material in the world, Mg alloys have been widely used in aeronautical and automotive industry. The Mg-RE (rare earth) alloys are outstanding for their extreme high strength and creep resistance, especially for the Mg-Gd-based alloys whose comprehensive performance at elevated temperature is superior to that of WE54 (Mg-5.4Y-2.3Nd-1.6Gd- 0.5Zr) and WE43 (Mg-4Y-3.3Nd-0.5Zr) alloys [1-4]. The mechanical properties of Mg-Gd alloys can be greatly enhanced by the thermal stable precipitates containing RE elements, which form during the aging process, but the coarse intermetallics in as-cast alloys distributing at grain boundaries are harmful [5-7]. This means that the intermetallics have a great influence on the comprehensive properties of alloys, especially for Mg-Gd-based alloys due to the various and complex intermetallics. So, it has a significant contribution to the preparation and heat treatment process of Mg-Gd alloys by investigating the growth behavior of intermetallics and diffusion kinetics in Mg-Gd binary system.
The investigations of Mg-Gd system have been performed for several times [8-10]. The latest Mg-Gd phase diagram reported by NAYEB-HASHEMI and CLARK [11] contains four intermetallics as Mg5Gd, Mg3Gd, Mg2Gd and MgGd. While Mg7Gd has been reported by NISHIJIMA et al [12] with an orthorhombic unit cell of a=0.64 nm, b=2.28 nm and c=0.52 nm. DAS et al [13] observed a layer of Mg4Gd6 formed at the interface of Mg/Gd when the diffusion couples were annealed at 743 K for 168 h. Furthermore, the thickness of each layer is almost proportional to the square root of the annealing time, which means that the growth of each layer is governed by the diffusion rate. XIAO et al [14] observed Mg7Gd without Mg4Gd6 after the diffusion couples annealed at 723 K for 384 h.
However, a limited number of investigations have been carried out on the reactive diffusion in Mg-Gd system above 773 K, and the thermodynamics and kinetic in the binary system are lack of understanding as well. Therefore, the diffusion couples of Mg-Gd binary system were annealed at 773 K for various time up to 48 h in high purity argon atmosphere in the present study. The growth behavior of the intermetallics was observed and the rate-controlling process of the reactive diffusion was analyzed on the basis of the observation.
2 Experimental
The Mg-Gd diffusion specimens were made of industrial pure Mg (99.99%, mass fraction) and Gd (99.99%, mass fraction), as shown in Fig. 1. Then, the Mg/Gd specimens were put in the vacuum heat treatment furnace filled with high purity argon. The specimens were isothermally annealed at 773 K for 12-48 h to make sure that all the intermetallic layers can be formed. Finally, the samples were took out from the heat treatment furnace, quenched quickly in cold water and cooled in air, respectively. The cross-sections of all the annealed diffusion couples were mechanically polished with 0.5 μm diamond suspension and cleaned with industrial pure alcohol.
The microstructures of the cross-section were observed by optical microscopy (OM) and scanning electron microscopy (SEM, JSM-6510). The concentration profiles of Gd through the interface were detected by electron probe microanalysis (EPMA, JXA-8230).
3 Results and discussion
3.1 Microstructures
The microstructures of the samples annealed at 773 K for 12-48 h quenched in cold water and cooled in air are shown in Figs. 2(a)-(c) and (d)-(f), respectively.

Fig. 1 Mg-Gd diffusion couple specimens
As can be seen, different intermetallic layers form at the Mg/Gd interface, and the total thickness of the intermetallics increases with increasing the annealing time. The typical backscattered electron (BSE) images for the Mg-Gd couples annealed at 773 K for 12 h are shown in Figs. 2(g) and (h). As can be seen, four different intermetallic layers form at the Mg/Gd interface. The composition of each intermetallic layer was measured by EPMA and the intermetallics are confirmed as Mg5Gd, Mg3Gd, Mg2Gd and MgGd, respectively. Mg2Gd and MgGd cannot be distinguished clearly in Fig. 2(g) for their small thickness. While in Fig. 2(h), Mg2Gd and MgGd are distinguished with the thickness of about 3 and 1 μm, respectively.
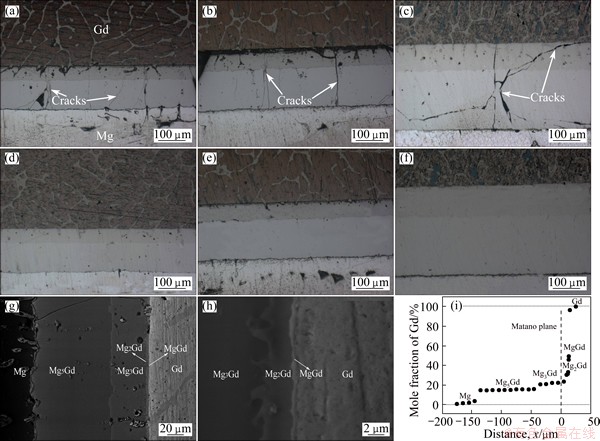
Fig. 2 Microstructures of samples annealed at 773 K for 12 h (a, d), 24 h (b, e) and 48 h (c, f) quenched in cold water (a-c) and cooled in air (d-f), BSE images of sample annealed at 773 K for 12 h (g, h) and concentration profile of Gd in sample annealed at 773 K for 12 h (i)
The concentration profile of Gd detected by EPMA on the cross-section of diffusion couple annealed at 773 K for 12 h is shown in Fig. 2(i). The solubility range of Gd in the four intermetallic layers Mg5Gd, Mg3Gd, Mg2Gd and MgGd are 14.2%-16.3%, 20.1%-25.5%, 33.6%-36.2% and 46.5%-48.9% (mole fraction), respectively. Meanwhile, the solubility of Gd in α-Mg is 3.4% (mole fraction) at 773 K as detected by EPMA in Fig. 2(i) and the latest experimental datum of the solubility of Gd in α-Mg reported by NAYEB- HASHEMI et al [11] is 3.5% (mole fraction) at 773 K. So, the experimental results agree well with the literature data. However, neither Mg7Gd nor Mg4Gd6 is observed in the present experiment, this may be the fact that different intermetallics can form under different experimental conditions [15,16]. The temperature in the present experiment is 773 K, while Mg7Gd and Mg4Gd6 were observed at 723 and 743 K, respectively.
The cracks are observed in all the samples annealed for various time up to 48 h in Figs. 2(a)-(c) but not in Figs. 2(d)-(f), and this can be attributed to the factor of quenching-induced stress. As all the intermetallics formed during annealing are brittle, when the samples were quenched in water, the quenching-induced stress may exceed the strength of the intermetallics, which will lead them to crack.
3.2 Growth behavior of intermetallics
The thicknesses of the intermetallic layers δi (i stands for the phases of Mg5Gd, Mg3Gd, Mg2Gd and MgGd, respectively) are evaluated by Eq. (1) at each annealing time [13,15,16] in BSE images like Figs. 2(g) and (h). The results are listed in Table 1, and as can be seen, the thickness δi monotonically increases with increasing the annealing time.
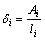 (1)
(1)
where Ai and li are the areas and lengths of intermetallic layers, respectively.
According to the growth kinetics for intermetallics in alloys, the layer thickness is proportional to the square root of time, as shown in Eq. (2), when the intermetallics growth is controlled by the diffusion rate.
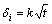 (2)
(2)
where k is the growth constant and t is the annealing time.
Table 1 Thickness of intermetallics formed in samples annealed at 773 K for 12-48 h
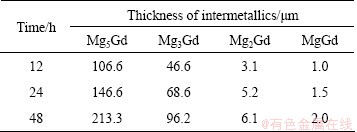
The nucleation time for the intermetallics is assumed to be negligible compared with the annealing time, namely δi=0 when t=0 and δi>0 when t>0. This is a necessary assumption to calculate the kinetics properly in the diffusion couple experiments. To prove the validity of the assumption, the thickness of each intermetallic layer against the square root of time is plotted in Fig. 3(a). As shown, the plotted points lie quite well in a straight line and the line intersects through the origin, which proves that the assumption is valid.
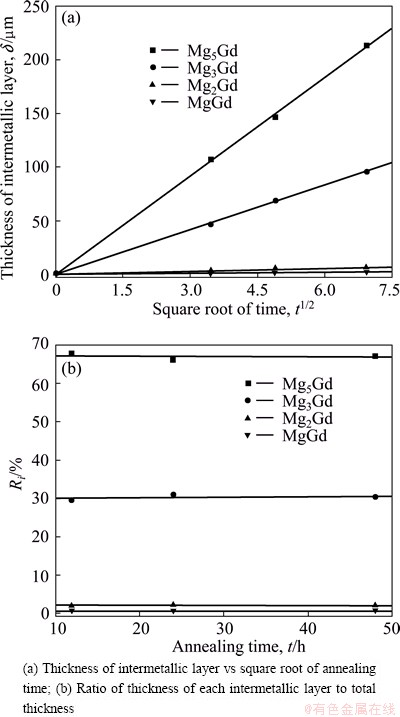
Fig. 3 Growth behavior of intermetallic layers at 773 K
The ratio of thickness of each intermetallic layer to the total thickness was calculated by Eq. (3) and the results are shown in Fig. 3(b). As can be seen, the Ri is almost constant, which means that the growth of each intermetallic layer is controlled by the same rate- controlling process at a certain temperature.
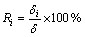 (3)
(3)
where Ri is the ratio of thickness of each intermetallic layer to the total thickness, δ is the total thickness of the intermetallics layers.
3.3 Diffusion kinetics in Mg-Gd binary system
To investigate the diffusion kinetic in Mg-Gd binary system, different diffusion coefficients of Gd in varying intermetallics should be calculated because four intermetallics formed during annealing.
The Matano plane was determined by the famous Heumann-Matano method, as shown in Fig. 4 and the diffusion coefficient of Gd in different intermetallic phases in Mg-Gd binary system can be calculated by Eq. (4) and the results are listed in Table 2.
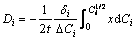 (4)
(4)
where Di and Ci are the interdiffusion coefficient (m2·s-1) and the concentration profile of solute in i phase, respectively, x is the distance from the Matano interface (m), t is the diffusion time (s), DCi is the concentration difference of solute between both ends of i phase and 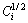 is the middle solute composition of the i phase.
is the middle solute composition of the i phase.
As shown in Table 2, the diffusion coefficient of Gd is varying in different intermetallics, and it decreases with increasing the solute content of intermetallics. This can be attributed to the driving force caused by varying concentration gradient between Gd and different intermetallics.
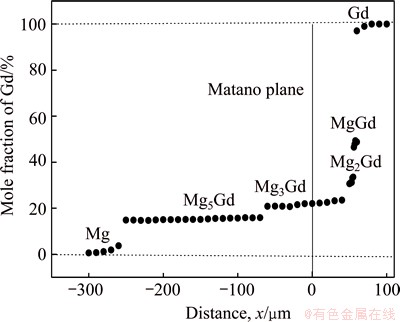
Fig. 4 Concentration profile of Gd in sample annealed at 773 K for 48 h
Table 2 Diffusion coefficients of Gd in different intermetallic phases at 773 K (10-12m2·s-1)

4 Conclusions
1) Four different intermetallic layers form at the Mg/Gd interface and they are Mg5Gd, Mg3Gd, Mg2Gd and MgGd, respectively.
2) The layer thickness δi (i stands for the phases of Mg5Gd, Mg3Gd, Mg2Gd and MgGd, respectively) monotonically increases with increasing the annealing time t according to equation: 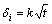 , which indicates that the growth behavior of the intermetallics is controlled by the diffusion rate.
, which indicates that the growth behavior of the intermetallics is controlled by the diffusion rate.
3) The diffusion coefficient of Gd in different intermetallics at 773 K was calculated by Matano method.
References
[1] LI Ji-lin, CHEN Rong-shi, MA Yue-qun. Effect of Zr modification on solidification behavior and mechanical properties of Mg-Y-RE (WE54) alloy [J]. Journal of Magnesium and Alloys, 2013, 1: 346-350.
[2] XIA Xiang-sheng, CHEN Qiang, LI Jian-ping, SHU Da-yu, HU Chuan-kai, HUANG Shu-hai, ZHAO Zu-de. Characterization of hot deformation behavior of as-extruded Mg-Gd-Y-Zn-Zr alloy [J]. Journal of Alloys and Compounds, 2014, 610: 203-208.
[3] MA Ming-long, ZHANG Kui, LI Xing-gang, LI Yong-jun, ZHANG Kang. Homogenization heat treatment of GWN751K magnesium alloy [J]. The Chinese Journal of Nonferrous Metals, 2010, 20(1): 1-9. (in Chinese)
[4] LI Yong-jun, ZHANG Kui, LI Xing-gang, ZHAO Xin, ZHANG Kai, ZOU Jing-bin, SONG Wei. Homogenizing and microstructure of Mg-Y-Nd-Zr alloy [J]. Chinese Journal of Rare Metals, 2008, 32(6): 679-683. (in Chinese)
[5] LI Ting. Study on the microstructure of EW75 alloy and its evolution during fabrication process [D]. Beijing: General Research Institute for Nonferrous Metals, 2013. (in Chinese)
[6] LI Ting, DU Zhi-wei, ZHANG Kui, LI Xing-gang, YUAN Jia-wei, LI Yong-jun, MA Ming-long, SHI Guo-liang. Morphology and crystallography of β precipitate phase in Mg-Gd-Y-Nd-Zr alloy [J]. Transaction of Nonferrous Metal Society of China, 2012, 22(12): 2877-2882.
[7] PENG Qiu-ming, MA Ning, LI Hui. Gadolinium solubility and precipitate identification in Mg-Gd binary alloy [J]. Journal of Rare Earths, 2012, 10: 1064-1069.
[8] MU Y L, WANG Q D, HU M L, JANIK V, YIN D D. Elevated-temperature impact toughness of Mg-(Gd, Y)-Zr alloy [J]. Scripta Materialia, 2013, 68: 885-890.
[9] ZHANG Xin-ming, WU Yi-ping, DENG Yun-lai, TANG Chang-ping. Constitutive equation during hot compression deformation of Mg-Gd-Y-Zr alloy [J]. The Chinese Journal of Nonferrous Metals, 2011, 21(12): 2987-2994. (in Chinese)
[10] HAMPL M, BLAWERT C, SILVACAMPOS M R, HORT N, PENG Q, KAINER K U, SCHMID-FETZER R. Thermodynamic assessment and experimental study of Mg-Gd alloys [J]. Journal of Alloys and Compounds, 2013, 581: 166-177.
[11] NAYEB-HASHEMI A A, CLARK J B. Phase diagrams of binary magnesium alloys [M]. Metal Park, OH: ASM International, 1988.
[12] NISHIJIMA M, HIRAGE K, YAMASAKI M, KAWAMURA Y. Characterization of β′ phase precipitates in an Mg 5at%Gd alloy aged in a peak hardness condition, studied by high-angle annular detector dark-field scanning transmission electron microscopy [J]. Materials Transactions, 2006, 47(8): 2109-2112.
[13] DAS S K, KANG Y B, HA T, JUNG I H. Thermodynamic modeling and diffusion kinetic experiments of binary Mg-Gd and Mg-Y systems [J]. Acta Materialia, 2014, 71: 164-175.
[14] XIAO Long, ZHONG Yan, CHEN Cui-ping, WULIU Ming-ming, LUO Tian-kuang, LIU Li-bin, LIN Kui. Isothermal section of Mg-Nd-Gd ternary system at 723 K [J]. Transaction of Nonferrous Metals Society of China, 2014, 24(3): 777-782.
[15] NAOI D, KAJIHARA M. Growth behavior of Fe2Al5 during reactive diffusion between Fe and Al at solid-state temperatures [J]. Materials Science and Engineering A, 2007, 459: 375-382.
[16] KAJIHARA M. Analysis of kinetics of reactive diffusion in a hypothetical binary system [J]. Acta Materialia, 2004, 52: 1193-1200.
Mg-Gd二元系在773 K下的反应扩散
郑伟文,李兴刚,张 奎,李永军,马鸣龙,石国梁
北京有色金属研究总院 有色金属材料制备加工国家重点实验室,北京 100088
摘 要:采用光学显微镜(OM),扫描电子显微镜(SEM)和电子探针(EPMA)等手段分析研究773 K下Mg-Gd二元系的反应扩散现象。结果表明:Mg-Gd扩散偶在773 K下热处理12~48 h后均形成四层明显的第二相扩散层,分别为Mg5Gd、Mg3Gd、Mg2Gd和MgGd相。各扩散层厚度δi(i分别代表Mg5Gd,Mg3Gd,Mg2Gd和MgGd相)与热处理时间t1/2呈正比,表明扩散层的生长由扩散速率所控制。同时各扩散层厚度占总扩散层厚度的比例基本保持不变,不随时间的延长而变化,这表明当热处理温度一定时,各扩散层的生长方式保持不变。最后利用Matano法计算Gd在不同相中的扩散系数。
关键词:Mg-Gd;反应扩散;热处理;金属间化合物
(Edited by Mu-lan QIN)
Foundation item: Projects (2013CB632202, 2013CB632205) supported by the National Basic Research Program of China
Corresponding author: Xing-gang LI; Tel: +86-10-82241163; E-mail: lixinggang1218@126.com
DOI: 10.1016/S1003-6326(15)64037-7
Abstract: The reactive diffusion in Mg-Gd binary system was studied at 773 K by optical microscopy (OM), scanning electron microscopy (SEM) and electron probe micro-analysis (EPMA). After annealing at 773 K for 12-48 h, four different intermetallic layers, Mg5Gd, Mg3Gd, Mg2Gd and MgGd, form at the Mg/Gd interfaces in the diffusion couples. The thicknesses of intermetallic layers δi (i stands for the phases of Mg5Gd, Mg3Gd, Mg2Gd and MgGd, respectively) are proportional to the square root of annealing time t1/2, which indicates that the growth behavior of the intermetallics is controlled by the diffusion rate. The ratio of thickness of each intermetallic layer to the total thickness is constant with increasing the annealing time, which means that the growth behavior is constant at a certain annealing temperature. The diffusion coefficient of Gd in different intermetallics was calculated by Matano method.


