文章编号:1004-0609(2008)11-2044-06
碳载Pd-Co-Au合金纳米粒子的制备及其电催化性能
王文明1, 2,李晓伟1,邹志清1,袁 婷1, 杜 翀1,夏保佳1,杨 辉1
(1. 中国科学院 上海微系统与信息技术研究所,上海 200050;
2. 湖南省电力勘测设计院,长沙 410007)
摘 要:
采用乙二醇还原法并进一步热处理制备碳载Pd-Co-Au (Pd-Co-Au/C)三元合金纳米电催化剂,通过旋转圆盘和环盘电极等技术评价催化剂对氧气还原反应的电催化活性,并分析氧气还原的机理。结果表明:合成Pd-Co-Au/C催化剂中Pd和Au两相面心立方(fcc)结构共存,且随着热处理温度的提高,对应于Au的衍射峰强度减弱,而Pd衍射峰强度增强;当温度高于800 ℃时,形成具有Pd单相fcc结构的三元合金纳米催化剂。氧气还原反应的动力学表明:氧气在Pd-Co-Au/C三元合金催化剂上按4e路径还原为水。电化学表征表明,在酸性介质中,经800 ℃热处理的Pd7Co2Au1/C催化剂对氧气还原的电催化活性最高,接近于商业化Pt/C的性能;而在含甲醇的酸性介质中,Pd-Co-Au/C催化剂电催化氧气还原的活性显著高于Pt/C。因此,Pd-Co-Au/C是一种高抗甲醇的新型氧气还原反应电催化剂。
关键词:
中图分类号:O643;O646 文献标识码:A
Preparation and electrocatalysis of carbon-supported
Pd-Co-Au ternary alloy nanoparticles
WANG Wen-ming1, 2, LI Xiao-wei1, ZOU Zhi-qing1, YUAN Ting1, DU Chong1, XIA Bao-jia1, YANG Hui1
(1. Shanghai Institute of Microsystem and Information Technology, Chinese Academy of Sciences,
Shanghai 200050, China;
2. Hunan Electric Power Design Institute, Changsha 410007, China)
Abstract: Carbon-supported Pd-Co-Au ternary alloy nanoparticle electrocatalysts were prepared by modified polyol reduction, and then heat-treated at 500-900 ℃. The electrocatalytic activities for the oxygen reduction reaction (ORR) were investigated based on porous rotating disk and disk-ring electrode techniques. The results show that the as-prepared Pd-Co-Au/C catalysts have broad reflections corresponding to the mixed Au and Pd face centred cubic (fcc) phases and the intensity of Au-rich phase relative to Pd-rich phase decreases with the increase of heat treatment temperature. When the heat treatment temperature is higher than 800 ℃, Pd ternary alloys with Pd fcc structure form. The electrochemical measurements indicate that the maximum catalytic activity for the ORR is found on the Pd7Co2Au1/C catalyst heat-treated at 800 ℃, which is close to the commercially available Pt/C. Kinetic analysis reveals that the ORR on Pd-Co-Au/C undergoes a four-electron process to water. Since Pd-Co-Au/C catalysts are inactive for the adsorption and oxidation of methanol, thus, they can be used as a new type of promising catalyst for methanol-tolerant ORR in a direct methanol fuel cell.
Key words: Pd alloy; nanoparticle; oxygen reduction reaction; electrocatalysis; methanol tolerance
直接甲醇燃料电池(DMFC)具有能量密度高,燃料来源丰富、储存与运输时安全性好,低污染,高能量转换效率和对价格的承受力较高等优点,因而有可能率先商业化[1]。然而,当前使用的铂阴极催化剂还存在两个主要问题:一是催化剂对氧气还原的电催化活性还不能满足商业化的要求;二是从阳极渗透到阴极的甲醇造成燃料的浪费和阴极“混合电位”效应,从而使电池约1/3的能量损失在阴极[2]。
当前,广泛研究抗甲醇的氧气还原电催化剂有Pt合金[3-4]、过渡金属大环化合物[5]、过渡金属硫化物[6]和Ru合金[7]等。Pt系列催化剂价格昂贵、资源有限、且抗甲醇性能差。后三者虽具有较高的催化活性和抗甲醇能力,但在无甲醇的介质中,催化氧还原的能力明显低于Pt/C催化剂,且长程稳定性差,制备成本也与Pt基催化剂不相上下。因此,研究兼具高活性和抗甲醇的新型阴极电催化剂具有非常重要的意义。
Pd较Pt来源丰富、价格便宜,且在酸性介质中对甲醇的吸附和氧化非活性,同时还具有较好的稳定性。因此,Pd及其合金对氧气还原的电催化及抗甲醇的氧气还原电催化引起了研究者的广泛兴趣[8-19]。PATTABIRAMAN[8]报道了碳载Pd催化剂在酸性介质中对氧还原的电催化性能。SAVADOGO等[9]首次采用Pd过渡金属合金作为氧还原的电催化剂,结果表明溅射沉积的Pd72Co28合金展现了比Pd更好的催化性能,其性能接近于Pt催化剂。而ADZIC等[10]则报道Pd2Co1/C具有比 Pt/C更高的电催化氧还原活性,同时还具有很高的抗甲醇性能。FERNANDEZ等[11-12]和RAGHUVEER等[13]初步研究了Pd-Co-Au/C和Pd-Co-Mo/C三元合金纳米催化剂用作质子交换膜燃料电池阴极催化剂的单电池性能。RAGHUVEER等[14]通过NaHB4还原和微乳法制备Pd7Co2Au1/C催化剂,并研究其用作氢氧质子交换膜燃料电池阴极催化剂的单电池性能。但他们都没有详细研究Pd-Co-Au和Pd-Co-Mo催化剂电催化氧气还原的机理及抗甲醇性能。同时,制备的Pd7Co2Au1/C和Pd7Co2Mo1/C三元合金催化剂粒径较大,经500~900 ℃热处理后,通过XRD计算所得的粒径分别为15~35 nm和24~42 nm。本文作者通过多元醇还原途径制备了粒径较小的Pd-Co/C合金纳米催化剂,获得的合金纳米催化剂对抗甲醇的氧气还原呈现较高的催化活性[15-16]。
根据文献[10-16]报道,Pd摩尔分数为60%~80%的Pd合金纳米催化剂具有较好的电催化氧还原性能和较高的抗甲醇性能。本文作者通过乙二醇还原途径制备Pd7Co2Au1/C和Pd6Co3Au1/C三元电催化剂,并在10% H2+90% N2(体积分数)气氛中热处理。对制得的催化剂进行了结构、电催化氧气还原活性和抗甲醇性能的表征,并分析了三元合金催化剂上氧气还原反应的机理。
1 实验
1.1 催化剂的制备
以PdCl2、CoCl2·6H2O和HAuCl4·4H2O(AR)为前躯体,炭黑(Vulcan XC-72, Cabot公司,BET比表面积为237 m2/g)为载体,采用调变的多元醇法[15-16]合成Pd质量分数为20%的Pd-Co-Au/C催化剂(Co和Au量按摩尔比计算)。然后在10%H2+90%N2(体积分数)气氛中于不同温度对合成催化剂进行热处理。
1.2 催化剂组成分析和X射线衍射表征
采用美国THERMO公司电感耦等离子体原子发散光谱(ICP-AES)对催化剂组成进行分析。催化剂结构采用日本理学D/MAX-2000 X射线衍射仪(XRD)进行测量,以Cu Kα为辐射源,滤玻片为Ni,扫描速度为2 (?)/min,扫描角度分辨率为0.02?。
1.3 电化学性能表征
多孔催化电极的制备程序为[3-4]:称取10 mg催化剂,加入2.5 mL超纯水、0.5 mL Nafion(浓度为5%, Aldrich)配成溶液,超声分散30 min,形成墨状浆液,用10 μL微量注射器吸取3 μL,滴涂在直径为3 mm的玻碳电极表面,室温自然晾干后,作为工作电极,电极表面Pd载量为28 μg/cm2。对电极为大面积的玻碳电极,参比电极为饱和甘汞电极,但本研究中提及的电位均相对于标准氢电极(RHE)电位。以20% Pt/C催化剂(Johnson-Matthey)作对比,标记为Pt/C。电化学测试在美国EG&G Model 273A恒电位仪和法国Radiometer旋转圆盘(RDE)/环盘电极(RRDE)上进行。电解液分别采用0.1 mol/L HClO4和0.1 mol/L HClO4+1 mol/L CH3OH溶液。实验在饱和氮气或氧气气氛中进行,所有电化学实验测试温度为(25±1) ℃。
2 结果与分析
2.1 催化剂组成和结构分析
图1所示为热处理前后Pd7Co2Au1/C和Pd6Co3Au1/C催化剂的XRD谱。由图1可见,热处理前后均没有出现Co及其氧化物的衍射峰,这是由于Co进入Pd fcc晶格形成Pd-Co合金[15-16],因此合成Pd-Co-Au/C催化剂只观察到归属于Au和Pd两相fcc结构的特征衍射峰。图1分别给出了对应的(111)、(200)和(220)晶面的衍射峰。随着热处理温度的增加,Au各晶面衍射峰明显向高角度方向移动,且强度变弱,而Pd各晶面的衍射峰强度明显增强;当热处理温度高于800 ℃时,只观察到归属于Pd fcc结构的晶面衍射峰,这是由于合成Pd-Co-Au/C催化剂中的Au均完全进入Pd-Co fcc晶格形成具有Pd单相fcc结构的合金,这表明了Pd-Co-Au/C三元合金纳米催化剂的形成。以(220)晶面为基础,根据Scherrer方程[15-16]计算晶粒大小。在相同热处理温度下,Pd6Co3Au1/C较Pd7Co2Au1/C具有相对小的粒径,与文献[17]的结果类似,且不同温度热处理Pd-Co-Au/C催化剂的粒径均比文献[12-14]中相同热处理温度下的粒径要小。Pd-Co-Au/C三元合金纳米粒子热处理前后的组成和结构表征结果如表1所列。

图1 Pd7Co2Au1/C和Pd6Co3Au1/C合金催化剂的XRD谱
Fig.1 XRD patterns of Pd7Co2Au1/C(a) and Pd6Co3Au1/C(b) alloy catalysts heat treated in 10%H2+90%N2 atmosphere at different temperatures
表1 Pd-Co-Au/C催化剂的组成、结构和电化学性能
Table 1 Composition, structure and electrochemical characteristics of as-prepared and heat-treated Pd-Co-Au/C catalysts

2.2 氧气在Pd-Co-Au/C催化剂上的电催化还原
图2所示为Pt/C、Pd-Co/C和Pd-Co-Au/C合金催化剂在氧气饱和0.1 mol/L HClO4溶液中的线扫描伏安曲线。由图2可见,催化剂电催化氧气还原的活性 顺序为:Pt/C>Pd7Co2Au1/C(800 ℃)>Pd6Co3Au1/C (800 ℃)>Pd7Co2Au1/C(750 ℃)>Pd7Co2Au1/C(900 ℃)>Pd2Co1/C(500 ℃)。800 ℃热处理Pd7Co2Au1/C较 800 ℃热处理Pd6Co3Au1/C对氧气还原呈现更高的电催化活性,更接近商业化Pt/C。在0.5 mA/cm2时,氧气在800 ℃ 热处理得到的Pd7Co2Au1/C上还原过电位仅比在Pt/C上增加约18 mV;在500~800 ℃范围内,Pd7Co2Au1/C电催化活性随热处理温度的升高而增强,活性的增强可归因于合金化程度的提高。虽然经900℃热处理后合金化程度进一步提高,但粒径也明显增大,因此经900 ℃热处理Pd7Co2Au1/C电催化氧气还原的活性反而有所降低。经800 ℃热处理Pd7Co2Au1/C具有比其它温度热处理的催化剂更高的电催化活性,可归因于合金化和粒径效应协同作用的结果。与文献[15-16]报道的Pd-Co/C氧气还原的活性相比,在0.5 mA/cm2时,氧气在800 ℃热处理Pd7Co2Au1/C上的还原过电位比在500 ℃热处理Pd2Co1/C催化剂上减小32 mV;而在Pd-Co/C催化剂中,500 ℃热处理Pd2Co1/C催化剂具有最高的电催化活性。这表明Au的引入显著增强了Pd-Co/C电催化氧气还原的活性。Au物种的作用可能是通过合金化调控了催化剂的电子效应,但具体原因还有待进一步研究。

图2 Pt/C、Pd-Co/C和Pd-Co-Au/C催化剂在氧气饱和HClO4溶液中的线扫描伏安曲线
Fig.2 Linear scanning voltammograms (LSVs) of Pt/C, Pd-Co/C catalyst and Pd-Co-Au/C alloy catalysts heat-treated at various temperatures in 0.1 mol/L HClO4 saturated with pure oxygen at scan rate of 5 mV/s and rotation speed of 1 600 r/min (Current density is normalized to geometric surface area. Ring currents, RRDE data for hydrogen peroxide production are contrasted)
据报道,氧气在Pd/C、Pd-Co/C和Pt/C电催化剂上的还原经历相似的的路径[9-10, 19],但Pd-Co-Au/C三元合金催化剂上氧气还原的机理还未见报道。图2中RRDE数据表明:当电位高于0.6 V时,环电流相对于盘电流可以忽略;当电位低于0.6 V时,环电流呈现增大的趋势,且Pd7Co2Au1/C上产生的H2O2量要稍高于Pt/C上。根据下式[7]计算H2O2产量:

计算表明,在DMFC单体有意义的工作电压范围即高于0.70 V时,氧气在Pd7Co2Au1/C和Pt/C催化剂上还原产生的H2O2量分别低于0.84%和0.22%。因此,Pd-Co-Au/C和Pt/C催化剂上氧气还原过程中产生H2O2量均可忽略不计,即氧气在Pd7Co2Au1/C和Pt/C上的还原经历相似的4e路径到最终产物水。
图3所示为经800 ℃热处理Pd7Co2Au1/C催化剂在氧气饱和的0.1 mol/L HClO4溶液中于不同旋转速度下的线扫描伏安曲线。由图3可看出,随旋转速度的增大,扩散控制区的极限电流密度依次增大,而动力学和混合控制区则基本重合。取图3中相同电位下不同转速ω-1/2对应的J -1作图,则可作出相应的Koutecky-Levich曲线。经800 ℃ 热处理Pd7Co2Au1/C催化剂的Koutecky-Levich曲线如图4所示。由图4可看出,不同电位下Pt/C和Pd7Co2Au1/C的Koutecky- Levich曲线基本平行,表明氧气在Pt/C和Pd7Co2Au1/C催化剂上的还原过程中转移的电子数相同。根据Koutecky-Levich方程[20] 还可以计算氧气在Pd7Co2Au1/C催化剂上的还原过程中转移的电子数n:

据文献[20],D = 1.9×10-5 cm2/s2,c = 1.18×10-6 mol/cm3,μ = 8.93×10-3 cm2/s。图4中各曲线的斜率即(0.62nFD2/3cμ-1/6)-1,由D、c和μ已知,则可根据斜率计算电子数n。计算表明,在0.2和0.4 V电位下,O2均经历4e路径还原为水,这与前面RRDE研究的结果相吻合。
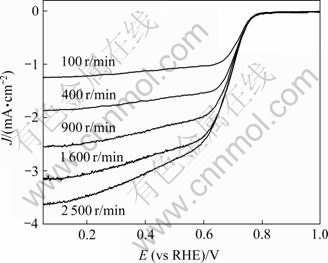
图3 Pd7Co2Au1/C催化剂在氧气饱和的HClO4溶液中的线扫描伏安曲线
Fig.3 LSVs of Pd7Co2Au1/C alloy catalyst heat-treated at 800 ℃ in 0.1 mol/L HClO4 saturated with pure oxygen at scan rate of 5 mV/s and different rotation speeds (Current density is normalized to geometric surface area)

图4 Pt/C和Pd7Co2Au1/C催化剂在HClO4 溶液中对氧还原反应的Koutecky-Levich曲线
Fig.4 Koutecky-Levich plots for ORR in 0.1 mol/L HClO4 solution on Pt/C and Pd7Co2Au1/C catalysts heat-treated at 800 ℃
2.3 Pd-Co-Au/C催化剂的抗甲醇性能
众所周知,在DMFC中,由于甲醇的渗透导致阴极商业化Pt/C电催化氧气还原的性能显著降低,造成电池约200~300 mV的电压损失,因此研制高抗甲醇的氧气还原电催化剂将加速DMFC商业化的步伐。图5所示为Pt/C和经800 ℃热处理Pd7Co2Au1/C与Pd6Co3Au1/C三元合金纳米催化剂在0.1 mol/L HClO4和0.1 mol/L HClO4+1 mol/L CH3OH溶液中的线扫描伏安曲线。由图5可见,曲线b相对曲线a和曲线d相对曲线c,均随电流密度的增大向低电位方向偏移程度略有增加,在0.5 mA/cm2时,分别仅向低电位方向偏移12 mV和19 mV,但DMFC单体有意义的工作电压范围即高于0.70 V时,曲线几乎没有向低电位方向的偏移。因此,Pd-Co-Au/C催化剂均具有很高的抗甲醇性能。而对Pt/C催化剂,曲线f相对曲线e显著向低电位方向偏移,增加约200 mV的过电位,且存在明显的甲醇氧化峰。因此,在含甲醇的酸性介质中,Pd-Co-Au/C电催化氧气还原的活性显著高于Pt/C,这表明Pd-Co-Au/C三元合金纳米粒子催化剂呈现很好的电催化氧气还原活性和高抗甲醇性能。
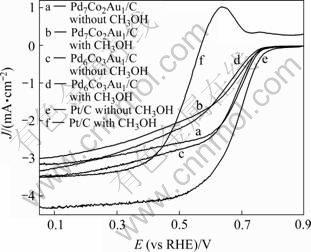
图5 Pt/C和Pd-Co-Au/C催化剂在HClO4 和HClO4+ CH3OH溶液中的线扫描伏安曲线
Fig.5 LSVs of Pt/C and Pd-Co-Au/C catalysts in 0.1 mol/L HClO4 solution and 1 mol/L CH3OH at scan rate of 5 mV/s and rotation speed of 1 600 r/min
3 结论
1) 采用乙二醇还原法并进一步热处理制备了Pd-Co-Au/C三元合金纳米电催化剂。
2) 合成Pd-Co-Au/C催化剂中Pd和Au两相fcc结构共存,随热处理温度的升高,Au进入Pd fcc晶格形成Pd单相fcc结构的合金纳米催化剂。
3) 氧气在Pd-Co-Au/C催化剂上经历4e路径还原为水。
4) 在酸性介质中,Pd-Co-Au/C催化剂电催化氧气还原的活性较Pd-Co/C催化剂更接近商业化Pt/C,Au的引入显著增强了Pd-Co/C电催化氧气还原的活性;在含甲醇的酸性介质中,Pd-Co-Au/C催化剂具有很高的抗甲醇性能,其电催化氧气还原的活性则显著高于商业化Pt/C。因此,Pd-Co-Au/C催化剂是一种高抗甲醇的新型氧气还原电催化剂。
REFERENCES
[1] ANTOLINI E, SALGADO J R C, GONZALEZ E R. Carbon supported Pt75M25 (M=Co, Ni) alloys as anode and cathode electrocatalysts for direct methanol fuel cells[J]. J Electroanal Chem, 2005, 580(1): 145-154.
[2] LEE K C, SAVADOGO O, ISHIHARA A, MITSUSHIMA S, KAMIYA N, OTA K. Methanol-tolerant oxygen reduction electrocatalysts based on Pd-3D transition metal alloys for direct methanol fuel cells[J]. J Electrochem Soc, 2006, 153(1): A20-A24.
[3] YANG Hui, VOGEL W, LAMY C, ALONSO-VANTE N. Structure and electrocatalytic activity of carbon-supported Pt-Ni alloy nanoparticles toward the oxygen reduction reaction[J]. J Phys Chem B, 2004, 108(30): 11024-11034.
[4] YANG Hui, ALONSO-VANTE N, LEGER J M, LAMY C. Tailoring, structure, and activity of carbon-supported nanosized Pt-Cr alloy electrocatalysts for oxygen reduction in pure and methanol-containing electrolytes[J]. J Phys Chem B, 2004, 108(6): 1938-1947.
[5] CHU D, JIANG R. Novel electrocatalysts for direct methanol fuel cells[J]. Solid state Ionics, 2002, 148(3/4): 591-599.
[6] REEVE R W, CHRISTENSEN P A, DICKINSON A J, HAMNETT A, SCOTT K. Methanol-tolerant oxygen reduction catalysts based on transition metal sulfides and their application to the study of methanol permeation[J]. Electrochim Acta, 2000, 45(25/26): 4237-4250.
[7] SCHMIDT T J, PAULUS U A, GASTERGER H A, ALONSO-VANTE N, BEHM R J. Oxygen reduction on Ru1.92Mo0.08SeO4, Ru/Carbon, and Pt/Carbon in pure and methanol-containing electrolytes[J]. J Electrochem Soc, 2000, 147(7): 2620-2624.
[8] PATTABIRAMAN R. Electrochemical investigations on carbon supported palladium catalysts[J]. Appl Catal A, 1997, 153(1/2): 9-20.
[9] SAVADOGO O, LEE K, OISHI K, MITSUSHIMA S, KAMIYA N, OTA K I. New palladium alloys for the oxygen reduction reaction in an acid medium[J]. Electrochem Commum, 2004, 6(2): 105-109.
[10] SHAO Min-hua, HUANG T, LIU P, ZHANG J, SASAKI K, VUKMIROVIC M B, ADZIC R R. Palladium monolayer and palladium alloy electrocatalysts for oxygen reduction[J]. Langmuir, 2006, 22(25): 10409-10415.
[11] FERNANDEZ J L, WALSH D A, BARD A J. Thermodynamic guidelines for the design of bimetallic catalysts for oxygen electroreduction and rapid screening by scanning electrochemical microscopy[J]. J Am Chem Soc, 2005, 127(1): 357-365.
[12] FERNANDEZ J L, RAGHUVEER V, MANTHIRAM A, BARD A J. Pd-Ti and Pd-Co-Au electrocatalysts as a replacement for platinum for oxygen reduction in proton exchange membrane fuel cells[J]. J Am Chem Soc, 2005, 127(38): 13100-13101.
[13] RAGHUVEER V, MANTHIRAM A, BARD A J. Pd-Co-Mo eletrocatalyst for the oxygen reduction reaction in proton exchange membrane fuel cells[J]. J Phys Chem B, 2005, 109(48): 22909-22912.
[14] RAGHUVEER V, FERREIRA P J, MANTHIRAM A. Comparison of Pd-Co-Au electrocatalysts prepared by conventional borohydride and microemulsion methods for oxygen reduction in fuel cells[J]. Electrochem Commum, 2006, 8(5): 807-814.
[15] WANG W M, ZHENG D, DU C, ZOU Z Q, ZHANG X G, XIA B J, YANG H, AKINS D L. Carbon-supported Pd-Co bimetallic nanoparticles as electrocatalysts for the oxygen reduction reaction[J]. J Power Sources, 2007, 167(3): 243-249.
[16] WANG W M, YANG H, CAO J Y, HU2NG Q H, ZOU Z Q, XIA B J. Carbon-supported Pd-Co alloy electrocatalysts for methanol tolerant oxygen reduction[J]. ECS Trans, 2007, 11(1): 379-1386.
[17] SHAO M H, SASAKI K, ADZIC R R. Pd-Fe nanoparticles as electrocatalysts for oxygen reduction[J]. J Am Chem Soc, 2006, 128(11): 3526-3527.
[18] ZHANG L, LEE K, ZHANG J. The effect of heat treatment on nanoparticle size and ORR activity for carbon-supported Pd-Co alloy electrocatalysts[J]. Electrochim Acta, 2007, 52(9): 3088-3094.
[19] TARASEVICH M R, CHALYKH A E, BOGDANOVSKAYA V A, KUZNETSOVA L N, KAPUSTINA N A. EFREMOV B N, EHRENBURG M R, REZNIKOVA L A. Kinetics and mechanism of oxygen reduction reaction at CoPd system synthesized on XC72[J]. Electrochim Acta, 2006, 51(21): 4455-4462.
[20] PAULUS U A. WOKAUN A, SCHERER G G, SCHMIDT T J, STAMENKOVIC V, RADMILOVIC V, MARKOVIC N M, ROSS P N. Oxygen reduction on carbon-supported Pt-Ni and Pt-Co alloy catalysts[J]. J Phys Chem B, 2002, 106(16): 4181-4191.
基金项目:国家自然科学基金资助项目(20673136);国家高技术研究发展计划资助项目(2006AA05Z136,2006AA04Z342);中国科学院“百人计划”择优支持资助项目;上海市浦江人才计划资助项目(06PJ14110)
收稿日期:2008-01-21;修订日期:2008-07-23
通讯作者:杨 辉,研究员,博士;电话:021-62511070;传真:021-32200534;E-mail: hyang@mail.sim.ac.cn
摘 要:采用乙二醇还原法并进一步热处理制备碳载Pd-Co-Au (Pd-Co-Au/C)三元合金纳米电催化剂,通过旋转圆盘和环盘电极等技术评价催化剂对氧气还原反应的电催化活性,并分析氧气还原的机理。结果表明:合成Pd-Co-Au/C催化剂中Pd和Au两相面心立方(fcc)结构共存,且随着热处理温度的提高,对应于Au的衍射峰强度减弱,而Pd衍射峰强度增强;当温度高于800 ℃时,形成具有Pd单相fcc结构的三元合金纳米催化剂。氧气还原反应的动力学表明:氧气在Pd-Co-Au/C三元合金催化剂上按4e路径还原为水。电化学表征表明,在酸性介质中,经800 ℃热处理的Pd7Co2Au1/C催化剂对氧气还原的电催化活性最高,接近于商业化Pt/C的性能;而在含甲醇的酸性介质中,Pd-Co-Au/C催化剂电催化氧气还原的活性显著高于Pt/C。因此,Pd-Co-Au/C是一种高抗甲醇的新型氧气还原反应电催化剂。


