Trans. Nonferrous Met. Soc. China 31(2021) 1484-1495
Effect of phosphogypsum on saline-alkalinity and aggregate stability of bauxite residue
Tao TIAN1, Chao-lan ZHANG2, Feng ZHU1, Shan-xin YUAN1, Ying GUO1, Sheng-guo XUE1
1. School of Metallurgy and Environment, Central South University, Changsha 410083, China;
2. School of Resources, Environment and Materials, Guangxi University, Nanning 530004, China
Received 23 May 2020; accepted 18 February 2021
Abstract:
A column experiment was conducted to investigate the effect of phosphogypsum (PG) on the saline- alkalinity, and aggregate stability of bauxite residue. Results showed that: with increasing leaching time, the concentrations of saline-alkali ions decreased while the  concentration increased in bauxite residue leachate; compared with CK (control group) treatment, pH, electric conductivity (EC), exchangeable sodium percentage (ESP), sodium absorption ratio (SAR), and exchangeable Na+ content of bauxite residue were reduced following PG treatment; average particle sizes in aggregates following CK and PG treatments were determined to be 155 and 193 nm, respectively. SR-μCT test results also confirmed that bauxite residue following PG treatment acquired larger aggregates and larger pore diameter. These results indicate that the PG treatment could significantly modulate the saline-alkalinity, and simultaneously enhance aggregate stability of bauxite residue, which provides a facile approach to reclaim bauxite residue disposal areas.
concentration increased in bauxite residue leachate; compared with CK (control group) treatment, pH, electric conductivity (EC), exchangeable sodium percentage (ESP), sodium absorption ratio (SAR), and exchangeable Na+ content of bauxite residue were reduced following PG treatment; average particle sizes in aggregates following CK and PG treatments were determined to be 155 and 193 nm, respectively. SR-μCT test results also confirmed that bauxite residue following PG treatment acquired larger aggregates and larger pore diameter. These results indicate that the PG treatment could significantly modulate the saline-alkalinity, and simultaneously enhance aggregate stability of bauxite residue, which provides a facile approach to reclaim bauxite residue disposal areas.
Key words:
bauxite residue; phosphogypsum; saline-alkalinity; aggregate stability;
1 Introduction
Bauxite residue, acting as a high alkaline waste, is generated during the production process from bauxite ore to alumina [1,2]. The land-based disposal areas, used to store large scale bauxite residue, usually produce harmful effects on the surrounding environment, including water and soil contamination [3,4]. High salinity and alkalinity of bauxite residue result in the difficulties of pedogenesis [5,6], further suppressing plants growth in bauxite residue disposal areas. Therefore, it is vital to adopt effective measures to regulate the saline-alkali properties of bauxite residue. Generally, pH, electrical conductivity (EC), sodium absorption ratio (SAR), and exchangeable sodium percentage (ESP) are the main parameters to evaluate the saline-alkali characteristics of bauxite residue [7]. These parameters are high in bauxite residue, i.e., pH: 9.2-12.8, EC: as high as ~28.4 mS/cm, ESP: 42%-80%, and SAR>30%, which are similar with the characteristics of saline-alkali soil [8-10]. Amendment application has been widely applied in saline-alkali soil to improve the physico-chemical properties and enhance fertilizer properties [11,12], which may be considered as a promising way to ecological remediation of the bauxite residue disposal areas, based on the similar characteristics between bauxite residue and saline-alkali soil.
Also, amendment application has a series of advantages, such as low cost, simple operation, easy transportation, and remarkable remediation effect in reducing saline-alkali characteristics of soil. Usually, gypsum and phosphogypsum were considered to be one of the promising candidates in the remediation of saline-alkali soil [7,12]. Gypsum addition into saline-alkali soil can elevate soil permeability by increasing ion concentration. With the increase of gypsum proportion, the significant decrease of EC and SAR in soil could be achieved [13]. ZHAO et al [12] investigated the effect of gypsum addition on the saline-alkali soil of Songnen plain, and found that the values of pH, EC, ESP, and SAR in saline-alkali soil are reduced by 14.6%, 38.6%, 61.2%, and 84.8% after two years, respectively. In addition, the aggregate stability also plays an important role in the pedogenesis process and saline-alkali modulation of bauxite residue [14,15]. Calcium ions of gypsum or phosphogypsum could displace sodium ions, which increases aggregate stability of saline-alkali soil, further improving the physical structure of soil [11,12]. However, so far, the bauxite residue studies in amendment application have been focused on only saline-alkalinity performance or single aggregate stability. Few reports on systematical investigation into amendment effect on the saline-alkali properties and aggregate stability of bauxite residue have been found [14].
The main goals in this research were to investigate variation of the saline-alkaline characteristics in bauxite residue following addition of amendments, reveal the effect of amendments on the evolution of aggregate structure, and inspect whether phosphogypsum can enhance remediation processes of bauxite residue.
2 Experimental
2.1 Sample characteristics
The tested bauxite residue was collected in a bauxite residue disposal area (surface layer 0-20 cm), which was located at Guangxi, China (24.075°N, 108.356°E). In this work, bauxite residue means a recently deposited sample which has been stacked about 60 d from the production to sample collection. The physical and chemical characteristics were recorded as follows: pH, EC, ESP, exchangeable Na content, exchangeable Ca content, exchangeable Mg content, and exchangeable K content showing 10.43, 197.30 μS/cm, 58.89%, 2.07×103 mg/kg, 1.26×103 mg/kg, 1.81 mg/kg, and 180.03 mg/kg, respectively. Phosphogypsum material was collected from a phosphate-fertilizer workshop at Yunnan Province, China, and its characteristics are as follows: the values of pH, EC, ESP, exchangeable Na content, exchangeable Ca content, exchangeable Mg content, and exchangeable K content are 2.75, 165.20 μS/cm, 5.89%, 122.09 mg/kg, 1.94×103 mg/kg, 41.29 mg/kg, and 160.48 mg/kg, respectively. Bauxite residue and phosphogypsum were air-dried for 7 d, and passed through a 2 mm sampling sieve prior to the incubation experiment.
2.2 Experiment design
The incubation experiment was carried out in a soil column (internal diameter of 12 cm, height of 100 cm) with several holes at the side wall, which was described in our previous work in detail [16]. Two treatments, including CK (control group, untreated bauxite residue) and PG (mixing with 2% phosphogypsum and bauxite residue), were set to fill the column at 0-25 cm (surface layer). From top to bottom, bauxite residue was uniformly filled to the column at 25-45 cm (middle layer) and 45-65 cm (sub-layer) for comparative study, followed by natural compaction. The bottom (>65 cm) of the column was padded with gauze to prevent the leakage of residue particles (Fig. 1). All of the treatments were in triplicate. In order to humidify bauxite residue samples, deionized water was continuously supplied from the bottom of the soil column until achieving the maximum water holding capacity of bauxite residue, staying for 48 h. Then, 175 mL water was calculated through the local average rainfall, leaching the residues from the top layer once every 2 d. A 500 mL conical bottle was placed at the bottom of the column to obtain the leachate every 3 d. The leaching process lasted for 30 d. At the end of the leaching process, residue samples of 0-25 cm, 25-45 cm, and 45-65 cm layers were collected, then the oven- dried process was carried out at 65 °C and the screened operation was conducted using a 2 mm sieve prior to physical and chemical analysis.
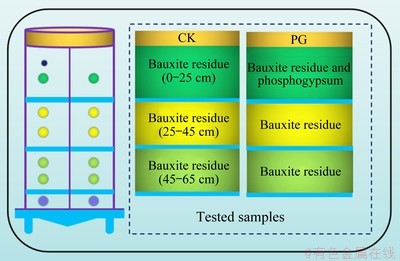
Fig. 1 Schematic diagram of experimental design
2.3 Characterization methods
2.3.1 Tested parameters
The tested parameters in leachates of bauxite residue included pH, EC, and concentrations of water-soluble ions (Na+, Ca2+, Mg2+, K+, 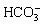 ,
, 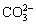 ,
,  , Cl-). Meanwhile, pH, EC, cation exchange capacities (CEC), ESP, SAR, exchange- able cation contents, and aggregate stability of bauxite residue were analyzed for all samples.
, Cl-). Meanwhile, pH, EC, cation exchange capacities (CEC), ESP, SAR, exchange- able cation contents, and aggregate stability of bauxite residue were analyzed for all samples.
2.3.2 Physico-chemical analysis
The values of pH and EC for all samples (solid/water mass ratio of 1:5) were determined by pH indicator (PHS-3C-pH, INESA, Shanghai, China) and conductivity meter (DDS-307, INESA, Shanghai, China), respectively [17]. Exchangeable cation contents were extracted with 1 mol/L ammonium acetate (pH=7), and were measured using inductively coupled plasma atomic emission spectrophotometry (ICP-AES). CEC value was determined by sodium acetate-flame photometry method [18]. Plasma emission spectrometer (ICPE-9800, Shimadzu, Japan) was used to examine the water-soluble cation concentrations (Na+, Ca2+, Mg2+, K+). Anion concentrations of 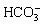 and
and 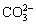 were measured by means of titration of sulfuric acid [19]. The concentration of Cl- was titrated by using AgNO3 solution, and
were measured by means of titration of sulfuric acid [19]. The concentration of Cl- was titrated by using AgNO3 solution, and  concentration was measured by BaCl2 addition treatment and titrated by means of ethylenediaminetetraacetate (EDTA) solution [19].
concentration was measured by BaCl2 addition treatment and titrated by means of ethylenediaminetetraacetate (EDTA) solution [19].
2.3.3 Micromorphology analysis of residue aggregates
The surface morphology and size of aggregate samples were obtained by a scanning electron microscope (SEM, FET Quanta-200). The powder samples were dispersed on the surface of copper sheet, and then small gold particles were sprayed on the top of samples to serve as a conductive coating. The element distribution was investigated using an energy dispersive spectrometer (EDS). The aggregate structure of bauxite residue was analyzed by synchrotron-related μCT (computer tomography) technique (beam line BL13W1 from Shanghai Synchrotron Radiation Facility Workstation (SSRF)). Aggregate samples were fixed in a plastic-based tube and mounted at a rotary stage, followed by rotating from 0° to 180° at an interval step of 0.1°, accompanying with scanning the samples with a photon energy of 19.5 keV. In total 1000 slices with 1200 × 1200 (pixels) in size were acquired. Image segmentation with an ImageJ 1.48 software was used to remove the obvious ring artifacts [20]. To refrain from edge effect, a volume with 300 × 300 × 300 (voxels) was extracted from the obtained 3D aggregates [21].
2.4 Data analysis
All data were expressed as their mean± standard deviation and were analyzed in Excel 2010 software. Figures were depicted by Origin 8.5 software.
3 Results and discussion
3.1 Saline-alkali characteristics of bauxite residue leachates
3.1.1 pH and EC
Figure 2(a) shows variation of pH value of bauxite residue leachates with different treatments. With the increase of leaching time, pH value of bauxite residue leachate decreased, which may be attributed to the changes of alkaline anions in bauxite residue leachates [8]. Compared with CK treatment, pH of bauxite residue leachate was reduced after PG application, because phospho-gypsum addition resulted in pH reduction of bauxite residue leaching due to releasing Ca2+ into solution, further causing precipitation of excessive anions (i.e., OH-, 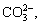 and
and 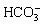 ) to generate Ca(OH)2, tri-calcium aluminate, hydrocalcumite, and CaCO3 [8,22]. As shown in Fig. 2(b), with increasing the leaching time, the decrease of EC of bauxite residue leachates occurred. EC value of bauxite residue leachate following PG treatment is higher than that of CK treatment, which indicates that phosphogypsum is beneficial to the enhancement salt elution rate [23].
) to generate Ca(OH)2, tri-calcium aluminate, hydrocalcumite, and CaCO3 [8,22]. As shown in Fig. 2(b), with increasing the leaching time, the decrease of EC of bauxite residue leachates occurred. EC value of bauxite residue leachate following PG treatment is higher than that of CK treatment, which indicates that phosphogypsum is beneficial to the enhancement salt elution rate [23].
3.1.2 Anions concentrations
As shown in Figs. 3(a, b), the concentrations of main anions (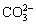 and
and 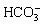 ) in leachates were inspected, which agreed with the previous results reported by JONES et al [23]. Compared with the CK treatment, after PG treatment, the concentrations of
) in leachates were inspected, which agreed with the previous results reported by JONES et al [23]. Compared with the CK treatment, after PG treatment, the concentrations of 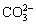 and
and 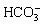 in bauxite residue leachate decrease, which can be ascribed to that precipitation occurs when Ca2+ from phosphogypsum suffers from
in bauxite residue leachate decrease, which can be ascribed to that precipitation occurs when Ca2+ from phosphogypsum suffers from 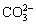 and
and 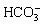 in bauxite residue leachates [10]. It can be observed that Cl- concentration of bauxite residue leachate after PG treatment is lower than that after CK treatment though Cl- concentration is low (Fig. 3(c)). Incorporating amendment into bauxite residue can lead to the reduction of Cl- concentration, which is in agreement with the result reported by LI et al [24]. Figure 3(d) shows that PG treatment elevates the
in bauxite residue leachates [10]. It can be observed that Cl- concentration of bauxite residue leachate after PG treatment is lower than that after CK treatment though Cl- concentration is low (Fig. 3(c)). Incorporating amendment into bauxite residue can lead to the reduction of Cl- concentration, which is in agreement with the result reported by LI et al [24]. Figure 3(d) shows that PG treatment elevates the  concentration of bauxite residue leachates. The main difference is that
concentration of bauxite residue leachates. The main difference is that  is introduced due to the dissolution of phosphogypsum (CaSO4·2H2O).
is introduced due to the dissolution of phosphogypsum (CaSO4·2H2O).
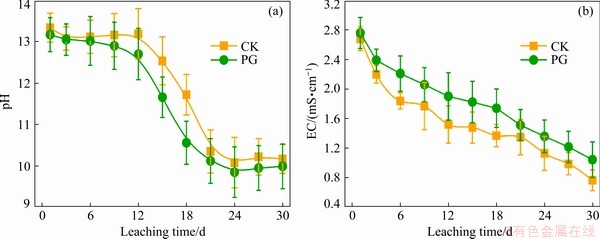
Fig. 2 Effects of different treatments on pH (a) and EC (b) of bauxite residue leachates (Bars were mean±standard deviation (SD, n=3))
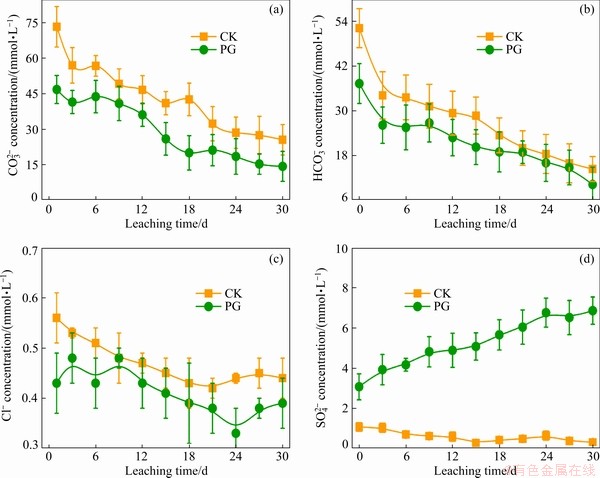
Fig. 3 Effects of different treatments on 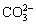 (a),
(a), 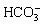 (b), Cl- (c), and
(b), Cl- (c), and  (d) concentrations of bauxite residue leachates
(d) concentrations of bauxite residue leachates
3.1.3 Cation concentrations
With increasing the leaching time, Na+ concentration of bauxite residue leachates was reduced (Fig. 4(a)). Also, Na+ concentration following PG treatment increased as compared to CK treatment. As shown in Fig. 4(b), with the increase of leaching time, Ca2+ concentration of bauxite residue leachates decreased. Moreover, compared with CK treatment, Ca2+ concentration of bauxite residue leachates after PG treatment decreased. This is because Ca2+ releases from the phosphogypsum addition, which can preferentially exchange Na+ [25], further releasing into bauxite residue leachates. In addition, phosphogypsum provides Ca2+ source which is easy to form CaCO3 precipitate with basic anion (i.e., 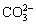 ) in bauxite residue [8], further resulting in the reduction of Ca2+ concentration of bauxite residue leachates. In this study, the variation of Ca2+ concentration is in consistent with
) in bauxite residue [8], further resulting in the reduction of Ca2+ concentration of bauxite residue leachates. In this study, the variation of Ca2+ concentration is in consistent with 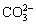 concentration of bauxite residue leachates (see Fig. 3(a)). As shown in Figs. 4(c, d), with increasing the leaching time, the concentrations of both Mg2+ and K+ are reduced although the Mg2+ concentration of bauxite residue leachates is lower. Similar cases occurred in previous research [23]. The above results indicated that amendment application has a positive effect on the mitigation of salinization of bauxite residue.
concentration of bauxite residue leachates (see Fig. 3(a)). As shown in Figs. 4(c, d), with increasing the leaching time, the concentrations of both Mg2+ and K+ are reduced although the Mg2+ concentration of bauxite residue leachates is lower. Similar cases occurred in previous research [23]. The above results indicated that amendment application has a positive effect on the mitigation of salinization of bauxite residue.
3.2 Saline-alkali characteristics of bauxite residue
3.2.1 pH and EC
The pH values for all treatments are presented in Fig. 5(a). Compared with CK treatment, pH value of the amended residues decreased at depths of 0-25, 25-45, and 45-65 cm. In the 0-25 cm layer, pH value following PG treatment decreased by 12.62%. Residue pH was mainly controlled by 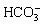 ,
, 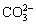 , and exchangeable Na+ [24]. Phosphogypsum addition provided excessive Ca2+ which could replace exchangeable Na+ in bauxite residue and react with
, and exchangeable Na+ [24]. Phosphogypsum addition provided excessive Ca2+ which could replace exchangeable Na+ in bauxite residue and react with 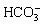 and
and 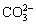 , further resulting in a decrease of pH [26]. Moreover, this result agreed with the previous research work reported by ZHAO et al [12], in which the pH value of saline-sodic soils was reduced by means of gypsum application. At 25-45 and 45-65 cm layers, compared with CK treatment, pH value following PG treatment exhibited reduction.
, further resulting in a decrease of pH [26]. Moreover, this result agreed with the previous research work reported by ZHAO et al [12], in which the pH value of saline-sodic soils was reduced by means of gypsum application. At 25-45 and 45-65 cm layers, compared with CK treatment, pH value following PG treatment exhibited reduction.
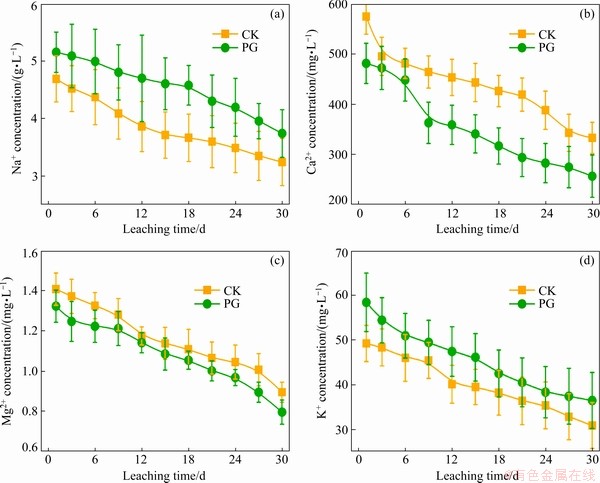
Fig. 4 Effects of different treatments on Na+ (a), Ca2+ (b), Mg2+ (c), and K+ (d) concentrations of bauxite residue leachates
Figure 5(b) shows the effect of amendments on the EC value of bauxite residue. EC value of bauxite residue following PG treatment obviously decreased as compared to CK treatment, at column depths of 0-25, 25-45, and 45-65 cm. In the 0-25 cm layer, compared with CK treatment, EC value decreased by 72.22% following PG treatment. This is probably because phosphogypsum caused reduction in Na content of bauxite residue, which is positively correlated with EC value [27]. However, amendment addition changed EC values of bauxite residue at 25-45 and 45-65 cm layers, which suggested that the amendment effect was mainly accumulated at 0-25 cm layer of bauxite residue.
3.2.2 Exchangeable cation contents
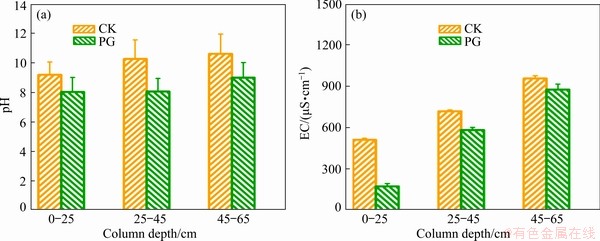
Fig. 5 Variation of pH (a) and EC (b) of bauxite residue at column depths of 0-25, 25-45, and 45-65 cm
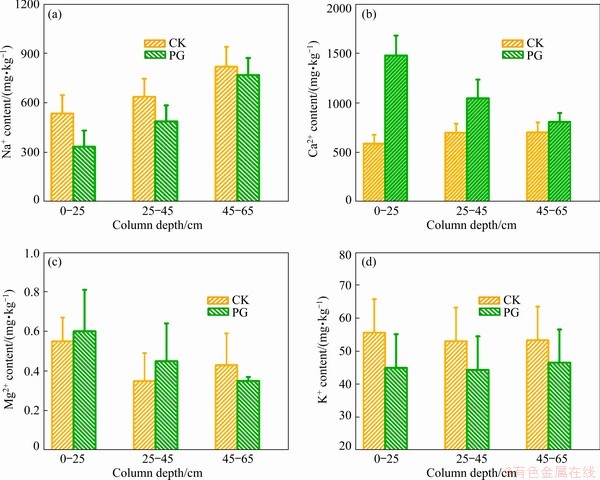
Fig. 6 Effects of different treatments on exchangeable Na+ (a), Ca2+ (b), Mg2+ (c), and K+ (d) contents of bauxite residue at column depths of 0-25, 25-45, and 45-65 cm
Amendment addition changed the contents of exchangeable cations (Na+, Ca2+, Mg2+, and K+) in bauxite residue at column depths of 0-25, 25-45, and 45-65 cm (Fig. 6). As presented in Fig. 6(a), for the same treatment, the exchangeable Na+ content increased with increasing column depth. As compared with CK treatment, exchangeable Na+ content following PG treatment decreased by 37.39% at the 0-25 cm layer, 23.51% at the 25-45 cm layer, and 6.24% at the 45-65 cm layer. As shown in Fig. 6(b), compared with CK treatment, PG treatment increased the content of exchangeable Ca2+ in bauxite residue. This phenomenon can be ascribed to the release of Ca2+ which has displacement with Na+ from the exchange complex system [26,28]. This result was also demonstrated by the EDS result in Fig. 7. In comparison with CK treatment, Na element distribution was sparse and dispersed, while Ca element exhibited a dense distribution following PG treatment. According to the EDS analysis, the amendment addition could increase content of exchangeable Ca2+ and reduce exchangeable Na+ content of bauxite residue. In comparison with CK treatment, at column depths of 0-25 and 25-45 cm, exchangeable Mg2+ content following PG treatment increased, but the similar trend was not obvious at 45-65 cm layer, as shown in Fig. 6(c). PG treatment decreased the content of exchangeable K+ in bauxite residue as compared to CK treatment, as shown in Fig. 6(d).
3.2.3 Alkaline characteristics
The variation of ESP of bauxite residue with different treatments is shown in Fig. 8(a). For the 0-25 cm layer, in comparison with CK treatment, the value of ESP with PG treatment decreased, and similar results were observed at 25-45 and 45-65 cm layers. This may be because the increase of ESP value of bauxite residue can reflect the high exchangeable Na+ content [29].
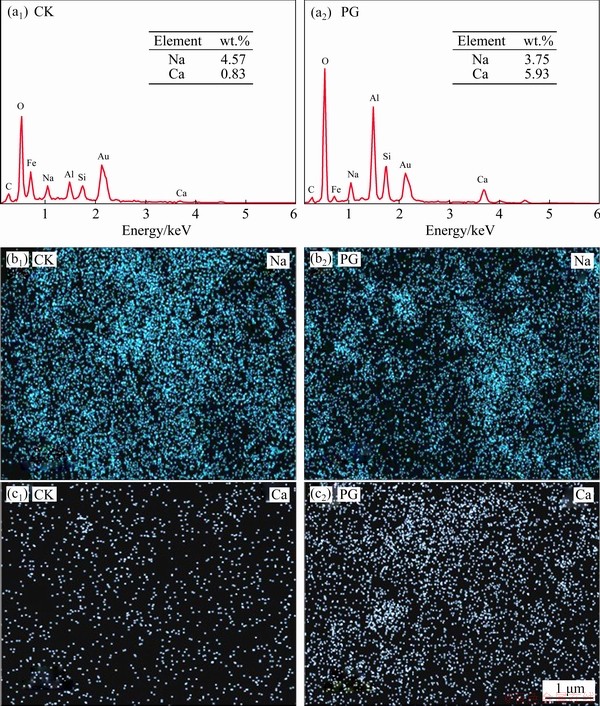
Fig. 7 EDS results showing effects of different treatments on contents and distributions of Na and Ca elements in bauxite residue
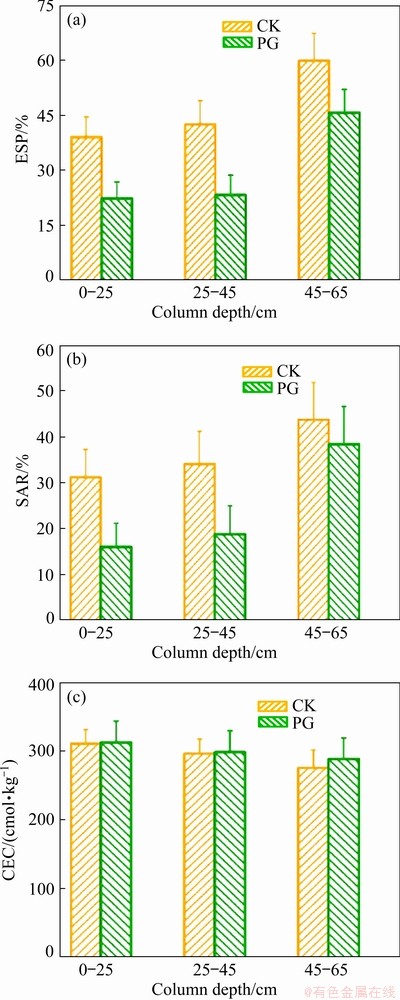
Fig. 8 Changes in ESP (a), SAR (b), and CEC (c) of bauxite residue at column depths of 0-25, 25-45 and 45-65 cm
SAR is one of the key parameters to inspect soil salinization [7,12]. The higher the SAR value is, the more toxic the soil will be [7]. The effect of amendment application on the SAR of bauxite residue is presented in Fig. 8(b). As compared to CK treatment, SAR decreased by 48.70%, 44.83%, and 12.15% after PG treatment at 0-25, 25-45, and 45-65 cm layers, respectively. Similar result was reported by CHAGANTI et al [7]. In their research, SAR value exhibited obvious reduction after leaching saline-sodic soils with the application of gypsum. In similarity with ESP, SAR reduction of bauxite residue may be induced by the decrease of Na+ content of bauxite residue, due to the exchange reaction between Na+ and Ca2+ generated from phosphogypsum [29]. The amendment effect on the CEC of bauxite residue is depicted in Fig. 8(c). For the 0-25 cm layer, in comparison with CK treatment, PG treatment enhanced CEC value of bauxite residue. However, amendment addition has insignificant influence on the CEC value of bauxite residue layers at column depths of 25-45 and 45-65 cm.
3.3 Micromorphology and structure of bauxite residue
The micro-aggregate characteristics of bauxite residue with different treatments were analyzed by scanning electron microscopy (SEM) observation, as displayed in Fig. 9. Most fine particles existed following CK treatment (Fig. 9(a1)). Following PG treatment, aggregate size increased in Fig. 9(a2), which indicated that PG treatment could effectively increase aggregate size, which suggested the improvement of aggregate structure and physical conditions of bauxite residue. The particle sizes from Figs. 9(b1, b2) were obtained in terms of the Gauss distribution fitting [30], as shown in Figs. 9(c1, c2). From the histogram, the average particle sizes of samples after CK and PG treatments were found to be 155 and 193 nm, respectively. To intuitively analyze the aggregate structure of bauxite residue following amendment application, the synchrotron irradiation technique was used to characterize the two-dimensional (2D) and three-dimensional (3D) structures of microaggregates, as shown in Fig. 10. Compared with CK treatment, bauxite residue following PG treatment had larger aggregate and pores, which is beneficial to plants growth in bauxite residue disposal areas [17]. ZHU et al [31] confirmed that larger aggregate size could improve physical structure and enhance aggregate stability of bauxite residue.
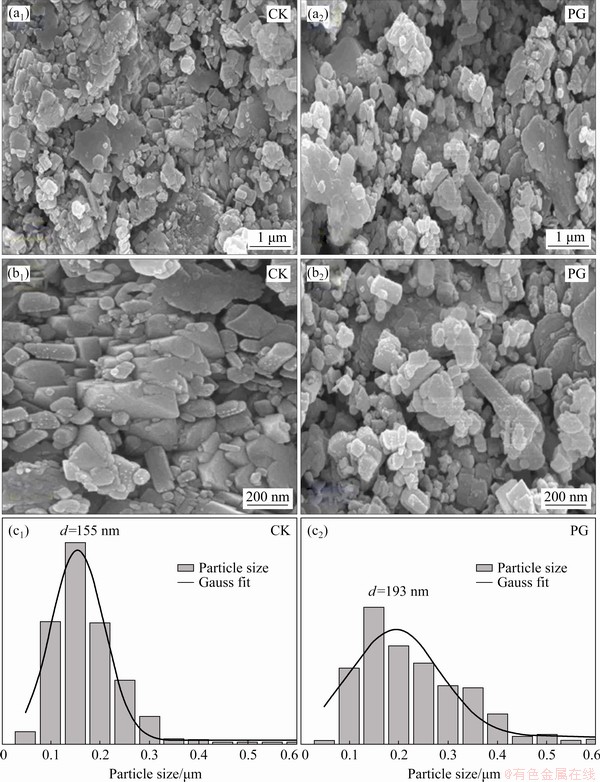
Fig. 9 Effects of different amendments on microstructures (a1, b1, a2, b2) and aggregate sizes (c1, c2) of bauxite residue
3.4 Potential mechanism for remediation of disposal areas
The above results and discussion suggested that phosphogypsum addition decreased the values of pH, EC, ESP, SAR, and exchangeable Na+ content of bauxite residue. By comparing the salinity, alkalinity, and aggregate stability of bauxite residue with different treatments, PG treatment exhibited superiority in reducing saline-alkali properties and enhancing aggregate stability. Figure 11 shows the effect of amendment (PG) on the saline-alkali properties of bauxite residue and the evolution of bauxite residue aggregate. On one hand, phosphogypsum acts as an industrial waste, and its main component is CaSO4·2H2O which contains lots of Ca2+. Therefore, partial precipitates (e.g., CaCO3) appear in bauxite residue when Ca2+ of phosphogypsum suffers from soluble alkalinity (i.e., 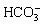 or
or 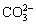 ) of bauxite residue [24], which induced pH reduction of bauxite residue. On the other hand, phosphogypsum can release large amount of Ca2+, which would displace the weak monovalent Na+ of bauxite residue from cation exchange sites [32,33]. Meanwhile, Na+ content of bauxite residue was positively correlated with EC [10,27] and SAR values [7]. Therefore, the decrease of Na+ content resulted in reduction of saline-alkalinity of bauxite residue. In addition, the bauxite residue aggregates following PG treatment acquired larger particle size and pore size from SEM and micro-CT, which enhanced aggregate stability of bauxite residue [20]. Our results demonstrated that PG treatment is a promising, controllable, and environment-friendly method for reducing the saline-alkali properties and enhancing aggregate stability of bauxite residue, which will be beneficial to vegetation reconstruction applications in bauxite residue disposal areas.
) of bauxite residue [24], which induced pH reduction of bauxite residue. On the other hand, phosphogypsum can release large amount of Ca2+, which would displace the weak monovalent Na+ of bauxite residue from cation exchange sites [32,33]. Meanwhile, Na+ content of bauxite residue was positively correlated with EC [10,27] and SAR values [7]. Therefore, the decrease of Na+ content resulted in reduction of saline-alkalinity of bauxite residue. In addition, the bauxite residue aggregates following PG treatment acquired larger particle size and pore size from SEM and micro-CT, which enhanced aggregate stability of bauxite residue [20]. Our results demonstrated that PG treatment is a promising, controllable, and environment-friendly method for reducing the saline-alkali properties and enhancing aggregate stability of bauxite residue, which will be beneficial to vegetation reconstruction applications in bauxite residue disposal areas.
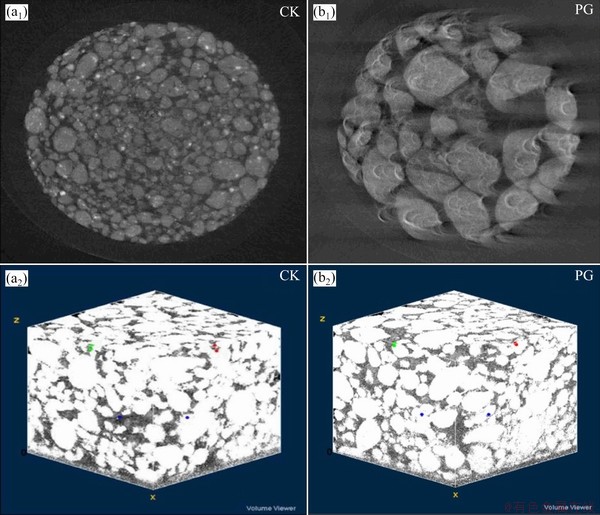
Fig. 10 Representative 2D slices (a1, b1) and 3D pore structures (a2, b2) of bauxite residue aggregates after different treatments
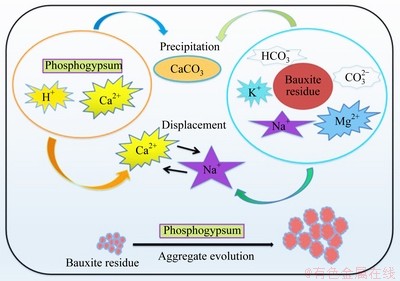
Fig. 11 Schematic diagram showing remediation mechanism of bauxite residue following phosphogypsum application
4 Conclusions
(1) With increasing leaching time, the parameters of saline-alkalinity in bauxite residue leachate were reduced except for  concentration.
concentration.
(2) Compared with CK treatment, pH value and the concentrations of 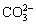 ,
, 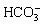 , Cl-, and Ca2+ decreased, while EC value and the concentrations of
, Cl-, and Ca2+ decreased, while EC value and the concentrations of  and Na+ of bauxite residue leachates increased after amendment applications.
and Na+ of bauxite residue leachates increased after amendment applications.
(3) In comparison with CK treatment, the values of pH, EC, ESP, SAR, and exchangeable Na+ content of bauxite residue were reduced, whilst aggregate size and pore structure of bauxite residue were improved following PG treatment.
(4) This study provided a low-cost effective strategy to control potential ecological risk in the disposal areas by amendment application, and demonstrated that PG treatment is conductive to pedogenesis process of bauxite residue.
Acknowledgments
The authors are grateful for the financial supports from the National Natural Science Foundation of China (Nos. 42030711, 41877511).
References
[1] LI Yi-wei, JIANG Jun, XUE Sheng-guo, MILLAR G J, KONG Xiang-feng, LI Xiao-fei, LI Ming, LI Chu-xuan. Effect of ammonium chloride on leaching behavior of alkaline anion and sodium ion in bauxite residue [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 2125-2134.
[2] COURTENY R, HARRINGTON T, BYRNE K A. Indicators of soil formation in restored bauxite residues [J]. Ecological Engineering, 2013, 58: 63-68.
[3] REN Jie, CHEN Juan, HAN Lei, WANG Mei, YANG Bin, DU Ping, LIU Fa-sheng. Spatial distribution of heavy metals, salinity and alkalinity in soils around bauxite residue disposal area [J]. Science of the Total Environment, 2018, 628-629: 1200-1208.
[4] HUO Qiang, LIU Xi, CHEN Li-jun, WU Yong-hong, WU Hai-yan, XIE Jian-ping, LIU Xin-xing, QIU Guan-zhou. Treatment of backwater in bauxite flotation plant and optimization by using box-behnken design [J]. Transactions of Nonferrous Metals Society of China, 2019, 29: 821-830.
[5] KONG Xiang-feng, JIANG Xing-xing, XUE Sheng-guo, HUANG Ling, HARTLEY W, WU Chuan, LI Xiao-fei. Migration and distribution of saline ions in bauxite residue during water leaching [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 534-541.
[6] DI CARLO E, BOULLEMANT A, COURTEY R. A field assessment of bauxite residue rehabilitation strategies [J]. Science of the Total Environment, 2019, 63: 915-926.
[7] CHAGANTI V N, CROHN D M, SIMUNEK J. Leaching and reclamation of a biochar and compost amended saline-sodic soil with moderate SAR reclaimed water [J]. Agricultural Water Management, 2015, 158: 225-265.
[8] GRAFE M, POWER G, KLAUBER C. Bauxite residue issues: III. Alkalinity and associated chemistry [J]. Hydrometallurgy, 2011, 108: 60-79.
[9] GOLORAN J B, PHILLIPS I R, CHEN Cheng-rong. Forms of nitrogen alter plant phosphorus uptake and pathways in rehabilitated highly alkaline bauxite processing residue sand [J]. Land Degradation Development, 2017, 28: 628-637.
[10] GRAFE M, KLAUBER C. Bauxite residue issues: IV. Old obstacles and new pathways for in situ residue bio- remediation [J]. Hydrometallurgy, 2011, 108: 46-59.
[11] MAHMOODABADI M, YAZDANPANAH N, SINOBAS L R, PAZIRA E, NESHAT A. Reclamation of calcareous saline sodic soil with different amendments (I): Redistribution of soluble cations within the soil profile [J]. Agricultural Water Management, 2013, 120: 20-38.
[12] ZHAO Yong-gan, WANG Shu-juan, LI Yan, LIU Jia, ZHUO Yu-qun, CHEN Hong-xiang, WANG Jing, XU Li-zhen, SUN Zhen-tao. Extensive reclamation of saline-sodic soils with flue gas desulfurization gypsum on the Songnen Plain, Northeast China [J]. Geoderma, 2018, 321: 52-60.
[13] QADIR M, QURESHI R H, AHMAD N. Reclamation of a saline-sodic soil by gypsum and Leptochloa fusca [J]. Geoderma, 1996, 74: 207-217.
[14] TIAN Tao, ZHOU Jing-ju, ZHU Feng, YE Yu-zhen, GUO Ying, HARTLEY W, XUE Sheng-guo. Effect of amendments on the leaching behavior of alkaline anions and metal ions in bauxite residue [J]. Journal of Environmental Sciences, 2019, 85: 74-81.
[15] ZHU Feng, HOU Jing-tao, XUE Sheng-guo, WU Chuan, WANG Qiong-li, HARTLEY W. Vermicompost and gypsum amendments improve aggregate formation in bauxite residue [J]. Land Degradation Development, 2017, 28: 2109-2120.
[16] TIAN Tao, KE Wen-shun, ZHU Feng, WANG Qiong-li, YE Yu-zhen, GUO Ying, XUE Sheng-guo. Effect of substrate amendment on alkaline minerals and aggregate stability in bauxite residue [J]. Journal of Central South University, 2019, 26: 393-403.
[17] DI CARLO E, CHEN Cheng-rong, HAYNES R J, PHILLIPS I R, COURTNEY R. Soil quality and vegetation performance indicators for sustainable rehabilitation of bauxite residue disposal areas: A review [J]. Soil Research, 2019, 57: 419-446.
[18] LU Ru-kun. Analytical methods of soil agricultural chemistry [M]. Beijing: Agricultural Science and Technology Press, 2000. (in Chinese)
[19] YU Pu-jia, LIU Shi-wei, YANG Hong-tao, FANG Gao-hua, ZHOU Dao-wei. Short-term land use conversions influence the profile distribution of soil salinity and sodicity in northeastern China [J]. Ecological Indicators, 2018, 88: 79-87.
[20] ZHU Feng, LIAO Jia-xin, XUE Sheng-guo, HARTLEY W, ZOU Qi, WU Hao. Evaluation of aggregate microstructures following natural regeneration in bauxite residue as characterized by synchrotron-based X-ray micro-computed tomography [J]. Science of the Total Environment, 2016, 573: 155-163.
[21] ZHOU Hu, PENG Xi, PETH S, XIAO Ti-qiao. Effects of vegetation restoration on soil aggregate microstructure quantified with synchrotron-based micro-computed tomography [J]. Soil and Tillage Research, 2012, 124: 17-23.
[22] COURTNEY R, KIRWAN L. Gypsum amendment of alkaline bauxite residue-plant available aluminium and implications for grassland restoration [J]. Ecological Engineering, 2012, 42: 279-282.
[23] JONES B E H, HAYNES R J, PHILLIPS R I. Cation and anion leaching and growth of Acacia saligna in bauxite residue sand amended with residue mud, poultry manure and phoshpogypsum [J]. Environmental Science and Pollution Research, 2012, 19: 835-846.
[24] LI Ya-ying, HAYNES R J, CHANDRAWANA I, ZHOU Ya-feng. Growth of Rhodes grass and leaching of ions from seawater neutralized bauxite residues after amendment with gypsum and organic wastes [J]. Journal of Environmental Management, 2019, 231: 596-604.
[25] XUE Sheng-guo, LI Meng, JIANG Jun, MILLAR G J, LI Chu-xuan, KONG Xiang-feng. Phosphogypsum stabilization of bauxite residue: Conversion of its alkaline characteristics [J]. Journal of Environmental Sciences, 2019, 77: 1-10.
[26] BURKE I T, PEACOCK C L, LOCKWOOD M B, RENFORTH P, GRUIZ K, MAYES W M. Behavior of aluminum, arsenic, and vanadium during the neutralization of red mud leachate by HCl, gypsum, or seawater [J]. Environmental Science Technology, 2013, 47: 6527-6535.
[27] KONG Xiang-feng, GUO Ying, XUE Sheng-guo, HARTLEY W, WU Chuan, YE Yu-zhen, CHENG Qing-yu. Natural evolution of alkaline characteristics in bauxite residue [J]. Journal of Cleaner Production, 2017, 143: 224-230.
[28] SAIFULLAH, DAHLAWI S, NAEEM A, RENGEL Z, NAIDU R. Biochar application for the remediation of salt-affected soil: Challenges and opportunities [J]. Science of the Total Environment, 2018, 625: 320-335.
[29] LI Ya-ying, HAYNES R J, CHANDRAWANA I, ZHOU Ya-feng. Properties of seawater neutralized bauxite residues and changes in chemical, physical and microbial properties induced by additions of gypsum and organic matter [J]. Journal of Environmental Management, 2018, 223: 489-494.
[30] YAO Chuang-ye, ISMAIL M, HAO Ai-ze, THATIKONDA S K, HUANG Wen-hua, QIN Ni, BAO Ding-hua. Annealing atmosphere effect on the resistive switching and magnetic properties of spinel Co3O4 thin films prepared by a sol-gel technique [J]. RSC Advances, 2019, 9: 12615-12625.
[31] ZHU Feng, LI Yu-bing, XUE Sheng-guo, HARTLEY W, WU Hao. Effects of iron-aluminium oxides and organic carbon on aggregate stability of bauxite residue [J]. Environmental Science and Pollution Research, 2016, 23: 9073-9081.
[32] XUE Sheng-guo, YE Yu-zhen, ZHU Feng, WANG Qiong-li, JIANG Jun, HARTLEY W. Changes in distribution and microstructure of bauxite residue aggregates following amendments addition [J]. Journal of Environmental Sciences, 2019, 78: 276-286.
[33] LI Xiao-fei, YE Yu-zhen, XUE Sheng-guo, JIANG Jun, WU Chuan, KONG Xiang-feng, HARTLEY W, LI Yi-wei. Leaching optimization and dissolution behavior of alkaline anions in bauxite residue [J]. Transactions of Nonferrous Metals Society of China, 2018, 28: 1248-1255.
磷石膏对赤泥盐碱性及团聚体稳定性的影响
田 桃1,张超兰2,朱 锋1,袁珊欣1,郭 颖1,薛生国1
1. 中南大学 冶金与环境学院,长沙,410083;
2. 广西大学 资源环境与材料学院,南宁 530000
摘 要:通过土柱实验研究磷石膏改良剂对赤泥盐碱性和团聚体稳定性的影响。结果显示:随着淋溶时间的延长,赤泥渗滤液中盐碱性离子浓度降低,而 浓度增加;与对照组相比,添加磷石膏可显著降低赤泥中pH、电导率、可交换钠百分比、钠吸收比和交换态Na+含量;对照组和磷石膏改良赤泥团聚体的平均颗粒尺寸分别为155和193 nm。同时,同步辐射微CT扫描结果也证明经磷石膏改良的赤泥获得更大的团聚体尺寸和孔径。以上结果表明,磷石膏改良可以显著调控赤泥的盐碱性,同时改善赤泥团聚体的稳定性,为赤泥堆场的修复提供一种经济有效的途径。
浓度增加;与对照组相比,添加磷石膏可显著降低赤泥中pH、电导率、可交换钠百分比、钠吸收比和交换态Na+含量;对照组和磷石膏改良赤泥团聚体的平均颗粒尺寸分别为155和193 nm。同时,同步辐射微CT扫描结果也证明经磷石膏改良的赤泥获得更大的团聚体尺寸和孔径。以上结果表明,磷石膏改良可以显著调控赤泥的盐碱性,同时改善赤泥团聚体的稳定性,为赤泥堆场的修复提供一种经济有效的途径。
关键词:赤泥;磷石膏;盐碱性;团聚体稳定性
(Edited by Wei-ping CHEN)
Corresponding author: Sheng-guo XUE, Tel: +86-13787148441, E-mail: sgxue@csu.edu.cn;
Feng ZHU, E-mail: zhufeng1990@csu.edu.cn
DOI: 10.1016/S1003-6326(21)65592-9
1003-6326/ 2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
2021 The Nonferrous Metals Society of China. Published by Elsevier Ltd & Science Press
Abstract: A column experiment was conducted to investigate the effect of phosphogypsum (PG) on the saline- alkalinity, and aggregate stability of bauxite residue. Results showed that: with increasing leaching time, the concentrations of saline-alkali ions decreased while the  concentration increased in bauxite residue leachate; compared with CK (control group) treatment, pH, electric conductivity (EC), exchangeable sodium percentage (ESP), sodium absorption ratio (SAR), and exchangeable Na+ content of bauxite residue were reduced following PG treatment; average particle sizes in aggregates following CK and PG treatments were determined to be 155 and 193 nm, respectively. SR-μCT test results also confirmed that bauxite residue following PG treatment acquired larger aggregates and larger pore diameter. These results indicate that the PG treatment could significantly modulate the saline-alkalinity, and simultaneously enhance aggregate stability of bauxite residue, which provides a facile approach to reclaim bauxite residue disposal areas.
concentration increased in bauxite residue leachate; compared with CK (control group) treatment, pH, electric conductivity (EC), exchangeable sodium percentage (ESP), sodium absorption ratio (SAR), and exchangeable Na+ content of bauxite residue were reduced following PG treatment; average particle sizes in aggregates following CK and PG treatments were determined to be 155 and 193 nm, respectively. SR-μCT test results also confirmed that bauxite residue following PG treatment acquired larger aggregates and larger pore diameter. These results indicate that the PG treatment could significantly modulate the saline-alkalinity, and simultaneously enhance aggregate stability of bauxite residue, which provides a facile approach to reclaim bauxite residue disposal areas.


