
Optimization of brine leaching of metals from hydrometallurgical residue
GUO Zhao-hui(郭朝晖), PAN Feng-kai(潘凤开), XIAO Xi-yuan(肖细元),
ZHANG Long(张 珑), JIANG Kai-qi(蒋凯琦)
Institute of Environmental Engineering, School of Metallurgical Science and Engineering,Central South University, Changsha 410083, China
Received 8 September 2009; accepted 19 January 2010
Abstract:
An orthogonal array, L16 (45 ), was used to examine the effects of four parameters, including NaCl concentration, H2SO4 concentration, temperature and pulp density, on the recovery of Cu, In, Pb and Zn from a hydrometallurgical residue via brine leaching. The results show that temperature of leaching solution has a significant effect on the recovery of Cu, In and Zn, while H2SO4 concentration has an obvious influence on these metals extraction. Both pulp density and NaCl concentration significantly affect Pb extraction. Based on the orthogonal array experiments, the optimum conditions for the extraction of Cu, In, Pb and Zn from hydrometallurgical residue are NaCl concentration of 250 g/L, H2SO4 concentration of 1.00 mol/L, temperature of 85 oC, and pulp density of 100 g/L. After 1 h of treatment at these optimum conditions, over 91% of the metals are extracted from the residue. Brine leaching is therefore suitable for the recovery of metals from hydrometallurgical residues.
Key words:
brine leaching; hydrometallurgical residue; valuable metal; optimization;
1 Introduction
The amount of solid wastes from the non-ferrous industrial activities, especially from Pb/Zn smelting activities, is increasing currently with industrialization and the rapid development of economy. The hydrometallurgical residues from smelting activities are often stockpiled and pose potential environmental risks due to the toxic elements, such as As, Cd, Cr, Cu, Ni, Pb, Sb and Zn which are contained in the residues[1]. With the rapid depletion of geogenic rich ore resources in recent years, it has become increasingly more financially attractive to recover valuable metals from previously stockpiled hydrometallurgical residues, while simultaneously reduce the potential of environmental pollution of toxic elements from the residues.
The recoveries of Ag, Cr, Cu, In, Pb, Zn, especially Pb and Zn, from the non-ferrous solid wastes were previously investigated through pyro- and hydro-metallurgical routes separately and in combination[2-10]. The lead-bearing materials derived from hydrometallurgical processing of zinc concentrate can be reacted with sodium-sulphide solution to recover Pb (90%-95%) and Ag (60%-70%)[2]. Significant amounts of Pb and Zn could also be recovered from zinc plant residues using a two-stage recovery process comprising roasting of the residue-H2SO4 mixture followed by a water and sodium chloride leaching of the residual solids[6]. With leaching conditions of 150 g/L H2SO4 (pH 2.5), 80-95 °C and pulp density of 120 g/L, 69.3%-71.9% Zn was extracted from zinc plant residue in 60-120 min[8-9]; up to 98.9% of Pb was extracted after 10 min of treatment at 300 g/L of NaCl concentration, 95 °C, pulp density of 50 g/L with 30 mL/L HCl addition[8]; while Pb extraction was 89.43% at pH 1.0, 300 g/L of NaCl concentration, 37 °C and stirring speed of 400 r/min after 30 min leaching[9]. Brine leaching, using NaCl, MgCl2 or CaCl2 as reagents, is generally the recognized method for the recovery of metal from non-ferrous solid wastes[2, 6-9, 11].
The effectiveness of the brine leaching method is based on the formation of complex metal chlorides in concentrated chloride solutions[9, 12]. For lead sulfate (PbSO4), for instance, the reactions in the brine leaching system can be illustrated by Eqs.(1)-(3)[11], in which insoluble PbSO4 is dissolved in brine solution by the formation of soluble PbCl42-:
![]()
![]()
![]() (1)
(1)
![]()
![]()
![]() (2)
(2)
![]()
![]()
![]() (3)
(3)
Similarly, the solubility of other metal residues (Au, Ag, Cu, Pb, Zn) can also be significantly enhanced via the formation of complex chlorides[12]. The process comprising Zn extraction with H2SO4 and Pb extraction with brine solution has been successfully demonstrated[6, 8-9], and consequently, acidic chloride solutions have been the common choice for increasing the recovery of metals when using a leaching system, for the recovery of Cu, Ni and Co[12], Cu, Ag and Zn[13], and so on. However, little attention has been drawn on the recovery of Cu, In, Pb and Zn in a brine leaching system.
The dissolution behavior of different metals from hydrometallurgical residues using an acidic brine solution is affected by many factors, such as concentration of NaCl[2, 6, 8-9], temperature[2, 8], pH or HCl volume[8-9], pulp density[2, 6, 8-9], reaction time[2, 8-9] and stirring speed[9]. Therefore, determination and optimization of the key factors are critically important for improving metal extraction efficiency. Orthogonal array design methodology is a simple and systematic approach used to determine the characteristic properties of key factors[14] and is widely applied[15-16]. In this study, the recovery of Cu, In, Pb and Zn from a hydrometallurgical residue using acidic brine solution was investigated using an orthogonal array design. The objectives of this study were to elucidate the contribution rates of NaCl concentration ρ(NaCl), H2SO4 concentration c(H2SO4), temperature and pulp density in the leaching system on the recovery of Cu, In, Pb and Zn from the hydrometallurgical residues using the L16(45) orthogonal array (with one blank column); and to optimize the process parameters for metal extraction from the hydrometallurgical residue using the brine leaching system.
2 Experimental
2.1 Hydrometallurgical residue characterization
The hydrometallurgical residue was collected from a zinc smelter in Guangxi province of China. The oven-dried residue was crushed with a stainless steel hammer and subsequently ground using a ball mill and sieved to be less than 120 ?m. Chemical composition of the residue was (mass fraction, %): As 0.21, Cu 0.35, Fe 10.55, In 0.60, Pb 6.75, S 1.68, Zn 6.54. Mineralogical characteristics showed that the hydrometallurgical residue was mainly composed of indium sulfate (In2(SO4)3), indium oxide (In2O3), zinc ferrites (ZnFe2O4), lead sulfate (PbSO4), goethite (FeO(OH)), zinc sulfide (ZnS), copper sulfide (CuS), quartz (SiO2), jarosite (K2Fe6(SO4)4(OH)12) and fluckite (CaMnH2 (AsO4)2·2H2O) (Fig.1).
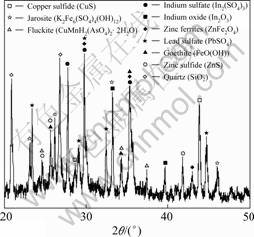
Fig.1 XRD pattern for hydrometallurgical residue
2.2 Orthogonal array and experimental procedure
Four important parameters in the brine leaching process, including (A) concentration of NaCl, (B) H2SO4 concentration, (C) temperature and (D) pulp density, were studied (Table 1). Orthogonal array of L16(45), which denotes five parameters each with four levels, was chosen to determine the effects of these four parameters on extraction efficiency of Cu, In, Pb and Zn, from the residue. In the L16(45) matrix, the fifth column was treated as a blank column. Each row represented a single experiment and each experiment was repeated twice to observe the effects of any noise. The order of levels was randomly chosen as the order of experiments to avoid any bias (Table 1). Possible interactions between variables were not considered significantly and were therefore not covered in the matrix, which focused on the main effects of the four independent parameters. Finally, the validity of this assumption was checked using the confirmation experiment with triplicate measures at optimum conditions[17].
Table 1 Experimental parameters and their levels for brine leaching
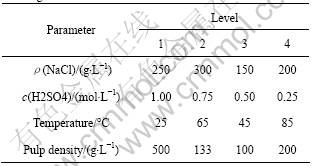
All experiments were conducted in 250 mL Erlenmeyer flasks, submerged in a thermostatic bath oscillator with digital temperature control (within ±1 °C). For each batch, 100 mL of leaching solution containing different predetermined concentrations of NaCl (150, 200, 250, 300 g/L) and H2SO4 (0.25, 0.50, 0.75, 1.00 mol/L) according to L16(45) matrix were introduced into the flasks and heated to the required temperature (25, 45, 65, 85 °C). Once reached the desired temperature, a predetermined amount of the residue (pulp density equals 100, 133, 200, 500 g/L) was added to the solution and the contents of the flask were stirred at 300 r/min using the bath oscillator. According to the preliminary tests performed, the leaching experiment was carried out for 1 h and the contents of the flask were collected in a centrifuge tube, vortexed briefly and centrifuged at 3 000 r/min for 5 min. The filtrate was stored at 4 °C before analysis.
2.3 Analytical and statistical methods
Mineralogical characterization of the hydrometallurgical residue was performed using an X-ray diffractometer (SIMENS2500X model, Germany). The concentration of Cu, Pb and Zn in the filtrate was analyzed using ICP-OES (Intrepid II XSP, USA). The concentration of indium (In) in the filtrate was determined using an atomic absorption spectrometer (WFX120 model, Japan) after extraction with 20 mL butyl acetate in 20 mL 5 mol/L HBr followed by stripping with 20 mL HCl of volume ratio 1?1[18].
Data were analyzed using Microsoft Excel 2003 and analysis of variance (ANOVA) was performed according to the method of NIAN et al[14].
3 Results and discussion
3.1 Parameters optimization for brine leaching
In the acidic brine system, four parameters, including the concentration of NaCl, H2SO4 concentration, temperature and pulp density, were investigated for the recovery of metals from the hydrometallurgical residue. The results of analysis variance for the effects of the four parameters on the recovery of metal from the residue are given in Table 2.
3.1.1 Concentration of NaCl
The concentration of NaCl significantly affected the recovery of both In and Zn with contribution rates of 10.1% and 11.4%, respectively (Table 2). For Pb extraction, the concentration of NaCl also had a highly significant effect and was the second important parameter (ηA =35.9%). In comparison, the F-value for concentration of NaCl on Cu recovery (F=0.81) was less than critical F-value for 95% confidence (F0.05=3.13), suggesting that for Cu recovery, the concentration of NaCl in the brine leaching was not important.
The extracted rate of In from the residue using an acidic brine solution increased with increasing concentration of NaCl. The maximum extracted rate of In was obtained at 200 g/L NaCl (Fig.2(a)). For Zn, the extraction rate was constant at approximately 70% when the concentration of NaCl ranged from 150 to 250 g/L. For Pb, when the concentration of NaCl changed from 150 to 250 g/L, the extraction rate sharply increased due to the formation of soluble lead chloro-complex PbCl42- (Eqs.(1)-(3)). However, the extraction rates of In, Pb and Zn dramatically decreased when concentration of NaCl exceeded 250 g/L due to the formation of sodium jarosite under acidic conditions and high concentration of NaCl. The formation of sodium jarosite which adsorbed onto the surface of the residue, would consequentially hinder the reactions between the liquid and the solid. For Cu, about 71% of Cu was extracted from the residue regardless of the NaCl concentration (Fig.2(a)). Since Pb2+ and Zn2+ were concentrated in the solution, the chloride ions were inclined to form ![]() and
and ![]() rather than
rather than ![]() in the acid brine leaching system. This result is in agreement with the order of equilibrium constant for the formation of metal chloro-complexes: Pb(II) (101-2) > Zn(II)(100-1) > Cu(II)(10-1-0)[12]. Therefore, the influence of NaCl concentration on Cu recovery was negligible in this study. The maximum recovery of the four metals considered from the residue could be obtained when the NaCl concentration in leaching solution was 250 g/L.
in the acid brine leaching system. This result is in agreement with the order of equilibrium constant for the formation of metal chloro-complexes: Pb(II) (101-2) > Zn(II)(100-1) > Cu(II)(10-1-0)[12]. Therefore, the influence of NaCl concentration on Cu recovery was negligible in this study. The maximum recovery of the four metals considered from the residue could be obtained when the NaCl concentration in leaching solution was 250 g/L.
Table 2 Results of analysis of variance for recovery of Cu, In, Pb and Zn from hydrometallurgical residue
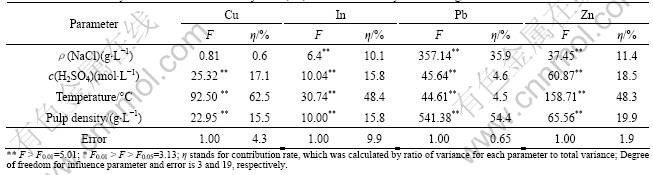
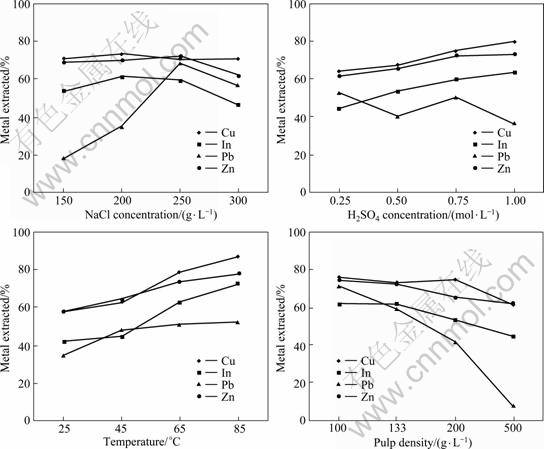
Fig. 2 Effect of NaCl concentration (a), H2SO4 concentration (b), temperature (c) and pulp density (d) on recovery of Cu, In, Pb and Zn from hydrometallurgical residue
3.1.2 H2SO4 concentration
The H2SO4 concentration has a significant effect on the recovery of both Cu and In (Table 2). The contribution rate of H2SO4 concentration was ηB=17.1% for Cu and ηB=15.8% for In and followed the same trend with temperature (ηC=62.5% for Cu and ηC=48.4% for In). The H2SO4 concentration also had a significant effect on the recovery of Pb and Zn, with contribution rates being 4.6% and 18.5%, respectively.
According to Eqs.(4)-(6), complexes, such as In2O3, ZnFe2O4, ZnS and CuS, are soluble in sulfuric acid solution[10]:
![]() =
=![]() (4)
(4)
![]() =
=![]() (5)
(5)
![]() =
=![]() (6)
(6)
The extracted rates of metal for Cu, In, and Zn in acidic brine solution increased with increasing H2SO4 concentration (Fig.2(b)). However, the extracted rate of Pb was variable, which is affected by the capture ability of chloride ions and sulfate ions (Eqs.(1))[11]. With high Cl- concentration, PbCl2 converts to PbCl3- and PbCl42- complexes according to Eqs.(2)-(3)[11]. The complexation equilibrium between chloride and lead ions
might be affected, for the solubility of PbSO4 is lower than that of PbCl2 (Ksp(PbSO4)=1.8×10-8 < Ksp(PbCl2)=1.2×10-5). The abundant chloride ion concentration, however, could bring about highly efficient extraction of Pb in the presence of concentrated H2SO4 according to Eqs.(1)-(3). For efficient recovery of Cu, In and Zn, 1.00 mol/L H2SO4 in the leaching solution was the best.
3.1.3 Temperature
According to the ANOVA analysis, temperature significantly affected the recovery of Cu, In and Zn from the residue with contribution rates of 62.5%, 48.4% and 48.3%, respectively (Table 2). For Pb recovery, temperature also exerted a significant effect, but the contribution rate was only 4.5%. The reactions of In2O3 (Eq.(4)) and ZnFe2O4 (Eq.(5)) with H2SO4 increase with increasing temperature[10], and the solubility of ZnS and CuS in acidic solution is also enhanced with increasing temperature. Therefore, to some extent, high temperature is useful for the recovery of Cu, In and Zn from the residue. In this study, about 42% In, 58% Zn and 58 % Cu were extracted at 25 °C, while about 73% In, 78% Zn and 86% Cu were extracted at 85 °C (Fig.2(c)). As observed for the other metals considered, the Pb recovered also increased when temperature ranged from 25 °C to 65 °C, but when the temperature was above 65 °C, the extraction efficiency of Pb only slightly increased due to the limited solubility of PbCl2. The maximum extraction of Cu, In and Zn from the residue occurred when the temperature of leaching solution was 85 °C.
3.1.4 Pulp density
The pulp density was a major dominant parameter of the extraction rate of Pb from the residue and the contribution rate of pulp density for Pb was 54.4% (Table 2). The pulp density also had a significant effect on the recovery of Cu, In and Zn with contribution rates of 15.5%, 15.8% and 19.9%, respectively. In general, the extraction rates of metals were inversely related to the pulp density (Fig.2(d)). For instance, for Pb, about 71% was extracted when the pulp density was 100 g/L, but only 8% was extracted when the pulp density was 500 g/L. This decrease in extraction rate with increasing pulp density was also obvious for In where about 62% In was extracted when the pulp density was 100 g/L, while about 44% In was extracted when the pulp density was 500 g/L. However, for Cu and Zn, the effect of pulp density is only slight. This result is not surprising since low pulp density provides sufficient hydrogen and chloride ions, and these ions would ensure thorough reactions between liquid and solid in the leaching system. A pulp density of 100 g/L obtained the maximum extraction rate of Cu, In, Pb and Zn, from the residue used in this study.
This systematic study showed that four parameters, including concentration of NaCl, H2SO4 concentration, temperature and pulp density, all had significant effects on the recovery of metals from a hydrometallurgical residue. Extractant solution temperature had the greatest impact on the recovery of In and Zn, while the effects of the other three parameters were equivalent for In and Zn extraction. For Pb extraction efficiency, pulp density and NaCl concentration were the two most crucial parameters, while the effects of H2SO4 concentration and temperature were less significant. For Cu, H2SO4 concentration, temperature and pulp density all had significant effects on the extraction rate while only the NaCl concentration did not affect the extraction efficiency of Cu. According to these findings and modeling of the significant effects by the orthogonal array design methodology, the optimum conditions for metal extraction were: ρ(NaCl) 250 g/L, c(H2SO4) 1.00 mol/L, temperature 85 °C and pulp density 100 g/L. The theoretical percentage extracted from the residue with a 95% confidence interval under these conditions was Cu (89 ± 6)%, In (87±12)%, Pb (89±6)% and Zn (94±4)% (Fig.3).
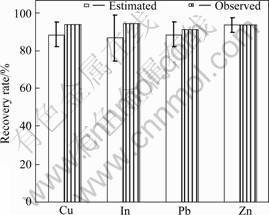
Fig.3 Recovery rate of Cu, In, Pb and Zn from hydrometallurgical residue under optimum conditions
3.2 Confirmation experiment
In this study, the experiment corresponding to the optimum conditions ρ(NaCl): 250 g/L, c(H2SO4): 1.00 mol/L, temperature 85 °C, pulp density 100 g/L) was carried out to verify the above predicted results. The extraction rate obtained during confirmation experiment is presented in Fig.3, and corresponds to 93.6% Cu, 94.1% In, 91.6% Pb and 93.2% Zn. As can be seen, there was good agreement between the predicted and the observed results, indicating that brine leaching is a possible technique to significantly increase the recovery of metals from hydrometallurgical residues under conditions that can be optimized from a few simple experiments using an orthogonal array. In addition, the residual leachant could be recycled within the brine leaching system after metals have been recovered to facilitate a more environmentally friendly process.
4 Conclusions
1) Using an orthogonal array design methodology, the recovery of Cu, In, Pb and Zn from a hydrometallurgical residue via brine leaching was studied. It is found that temperature is a crucial parameter for Cu, In and Zn extraction, with contribution rates of 62.5%, 48.4% and 48.3%, respectively. Pb extraction is significantly affected by pulp density and NaCl concentration, with contribution rates for Pb of 54.4% and 35.9%, respectively.
2) Pulp density and H2SO4 concentration also have significant effects on the recovery of Cu, In and Zn while H2SO4 concentration only slightly affects Pb recovery.
3) The optimum leaching conditions to the maximum extraction of Cu, In, Pb and Zn from the residue occur at ρ(NaCl) 250 g/L, c(H2SO4) 1.00 mol/L, temperature 85 °C, pulp density 100 g/L. After 1 h of treatment at the optimum conditions, the extraction rates from the residue are Cu 93.6%, In 94.1%, Pb 91.6% and Zn 93.2%. Brine leaching is suitable for the recovery of Cu, In, Pb and Zn from the hydrometallurgical residues.
References
[1] MANZ M, CASTRO L J. The environmental hazard caused by smelter slags from the Sta. Maria de la Paz mining district in Mexico [J]. Environmental Pollution, 1997, 98(1): 7-13.
[2] RAGHAVAN R, MOHANAN P K, PASTNAIK S C. Hydrometallurgical processing of lead-bearing materials for the recovery of lead and silver as lead concentrate and lead metal [J]. Hydrometallurgy, 2000, 58(2): 103-116.
[3] HOFFMANN G, SCHIRMER M, BILITEWSKI B, KASZ?S SAVOS M. Thermal treatment of hazardous waste for heavy metal recovery [J]. Journal of Hazardous Materials, 2007, 145(3): 351-357.
[4] ABDEL BASIR S M, RABAH M A. Hydrometallurgical recovery of metal values from brass melting slag [J]. Hydrometallurgy, 1999, 53(1): 31-44.
[5] LI Shi-qing, TANG Mo-tang, HE Jing, YANG Sheng-hai, TANG Chao-bo, CHEN Yong-ming. Extraction of indium from indium-zinc concentrates [J]. Transactions of Nonferrous Metals Society of China, 2006, 16(6): 1448-1454.
[6] TURAN M D, ALTUNDO?AN H S, T?MEN F. Recovery of zinc and lead from zinc plant residue [J]. Hydrometallurgy, 2004, 75(1/4): 169-176.
[7] ABDOLLAHI P, MORADKHANI D, BEHNIAN D. Lead recovery from Iranian zinc plant residue using brine leaching method [C]//XXIII International Mineral Processing Congress (IMPC). Istanbul, Turkey, 2006: 1515-1520.
[8] RU?EN A, SUNKAR A S, TOPKAYA Y A. Zinc and lead extraction from ?inkur leach residues by using hydrometallurgical method [J]. Hydrometallurgy, 2008, 93(1/2): 45-50.
[9] MORADKHANI D, FARAHMAND F, SAFARZADEH M S. Brine leaching of lead-bearing zinc plant residues: Process optimization using orthogonal array design methodology [J]. Hydrometallurgy, 2009, 95(3/4): 316-324.
[10] JHA M K, KUMAR V, SINGH R J. Review of the hydrometallurgical recovery of zinc from industrial wastes [J]. Resources, Conservation and Recycling, 2001, 33(1): 1-22.
[11] SINADINOVI? D, KAMBEROVI? ?, ?UTI? A. Leaching kinetics of lead from lead (II) sulphate in aqueous calcium chloride and magnesium chloride solutions [J]. Hydrometallurgy, 1997, 47(1): 137-147.
[12] PARK K H, DEBSISH M, KIM H I, GUO Xue-yi. Dissolution behavior of a complex Cu-Ni-Co-Fe matte in CuCl2-NaCl-HCl leaching medium [J]. Separation and Purification Technology, 2007, 56(3): 303-310.
[13] NU?EZ C, ESPIELL F, ROCA A. Recovery of copper, silver and zinc from Huelva (Spain) copper smelter flue dust by a chloride leach process [J]. Hydrometallurgy, 1985, 14(1): 93-103.
[14] NIAN C Y, YANG W H, TRANG Y S. Optimization of turning operations with multiple performance characteristics [J]. Journal of Materials Processing Technology, 1999, 95(1/3): 90-96.
[15] ?OPUR M, OZMETIN C, OZMETIN E, KOCAKERIM M M. Optimization study of the leaching of roasted zinc sulphide concentrate with sulphuric acid solutions [J]. Chemical Engineering and Processing, 2003, 43(8): 1007-1014.
[16] GUO Zhao-hui, CHENG Yi, QIU Guan-zhou, LIU Xue-duan, PAN Feng-kai. Optimization on bioleaching of metal values from Pb/Zn smelting slag [J]. The Chinese Journal of Nonferrous Metals, 2008, 18(5): 923-928. (in Chinese)
[17] SAFARZDEH M S, MORADKHANI D, ILKHCHI M O, GOLSHAN N H. Determination of the optimum conditions for the leaching of Cd–Ni residues from electrolytic zinc plant using statistical design of experiments [J]. Separation and Purification Technology, 2008, 58(3): 367-376.
[18] SUN Shu-yuan, SUN Ling-gao, YIN Qi-xi. Analysis handbook for non-ferrous metals and ores [M]. Beijing: Metallurgical Industry Press, 2008: 168-170. (in Chinese)
Foundation item: Project(20507022) supported by the National Natural Science Foundation of China
Corresponding author: Tel: +86-731-88836442; Fax: +86-731-88710171; E-mail: zhguo@mail.csu.edu.cn
DOI: 10.1016/S1003-6326(09)60408-8


