
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 2179-2183
Tortuosity factor for porous FeAl intermetallics fabricated by reactive synthesis
GAO Hai-yan1, HE Yue-hui1, ZOU Jin2, XU Nan-ping3, C.T. LIU 4
1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. Centre for Microscopy and Microanalysis, The University of Queensland, QLD 4072, Australia;
3. Membrane Science and Technology Research Center, Nanjing University of Technology, Nanjing 210009, China;
4. Department of Mechanical Engineering, The Hong Kong Polytechnic University, Hong Kong, China
Received 22 November 2011; accepted 29 February 2012
Abstract:
The tortuosity factor is the most critical parameter for the pore characteristic of porous materials. The tortuosity factor for porous FeAl intermetallics was studied based on the Darcy law and Hagen-Poiseuille equation. Porous stainless steel with the same pore structure parameter as porous FeAl was fabricated by powder metallurgy method for comparison. The results show that the tortuosity factor of porous FeAl intermetallics is smaller than that of porous stainless steel when their pore structure parameters are the same. The average tortuosity factor is 2.26 for the porous FeAl material and 2.92 for the porous stainless steel, calculated by Hagen-Poiseuille equation. The reason of the different tortuosity factors for porous FeAl and porous stainless steel was also explored through studying the pore formation mechanisms of the two types of porous materials.
Key words:
FeAl; intermetallics; porous material; tortuosity factor;
1 Introduction
Porous Fe-Al intermetallics have been developed recent years by reactive synthesis based on the Kirkendall effect using Fe and Al powders as raw material [1,2]. It was well documented that the transport properties of porous materials are based on the degree of tortuosity which determines the resistance to the transport of fluid molecules through the porous materials [3,4]. Therefore,it is important to estimate the transport properties of the new type of porous inorganic materials-porous FeAl, and it is necessary to explore its tortuosity factor.
However,the porous material is generally regarded as a black box [3] and the tortuosity factor of pores cannot be measured experimentally [5,6]. In recent studies, the pore structure has been characterized by simple parameters such as porosity, pore size and average length, in rare cases with average pore tortuosity [7,8]. In some studies, pore tortuosity has been estimated from soil hydraulic characteristics such as the hydraulic conductivity and the water retention characteristics[9]. ZHOU [10] described tortuosity factor of carbon nanotubes by high resolution transmission electron microscopy (HRTEM) and FAN [11] got the tortuosity factor by dynamic state method—ingle pellet string reactor method (SPSRM), whereas these methods are complex and data processing is generous. BISWAS and WINOTO [3] estimated tortuosity factor based on the Hagen- Poiseuille equation, which is a simple and easy performing method. No research of the tortuosity factor for porous FeAl has been carried out.
In this study, the tortuosity factor of the porous FeAl was explored based on the Darcy law and Hagen-Poiseuille (H-P) equation. The tortuosity factor of porous stainless steel with the same pore structural parameters as porous FeAl was also been studied for comparison.
2 Experimental
The porous FeAl material was fabricated as follows: 99.8% purified Fe and Al powders with the particle sizes of 3-5 μm were used as raw materials. The mixture of Fe and Al powders with the mole ratio of Fe to Al of about 6:4 was blended in a cylinder mixer. No lubricant was added in the mixture to maintain a sound metal-metal contact. The compact discs with the diameter of 32 mm were cold pressed (under the pressure of 250 MPa) and sintered in vacuum. The step heating method [1] was used for the sintering. The final sintering was carried out at 1000 ℃ for 60 min. For comparison, porous stainless steels were fabricated using stainless steel alloy powders (3-8 μm) as raw materials. The stainless steel compact discs with the diameter of 32 mm were cold pressed (under the pressure of 200 MPa to gain identical pore structures as the porous FeAl materials) and sintered in a vacuum with the finally sintering temperature of 950 ℃ for 30 min. A series of thicknesses of compact discs for the two different porous materials were manufactured for the permeability study.
The porosity of resultant porous FeAl and stainless steel materials was measured by the Archimedes water-immersion method. Their microstructures were characterized by a scanning electron microscope (SEM, LEO1525) operated at 20 kV. The gas permeability and pore size were determined on a FBP-3III porous material test instrument,and 99.9% purified N2 was used as the fluid medium. The permeability (K) of the porous materials was evaluated using equation (1):
![]() (1)
(1)
where Q is the flow rate; Δp is the pressure drop from entrance to exit of the sample; A is the effective cross-sectional area of the sample.
The gas permeametry equipment used in this study is shown in Fig. 1. The sample was fixed in the center of a stainless steel mold. Δp and Q were measured using this equipment. The permeability is calculated from the slope of the line plotted for Δp versus Q using equation (1).
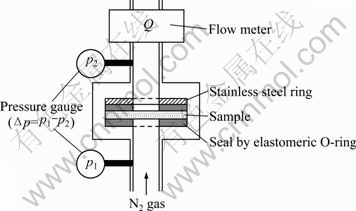
Fig. 1 Schematic model of equipment for gas permeametry
3 Results and discussion
3.1 Volume change of porous materials after sintering
Traditional porous metallic materials fabricated by powder metallurgy usually include porous Ti, Ni and stainless steel (SS) materials. The volume changes after sintering of these porous materials and porous FeAl material are shown in Fig. 2. As can be seen, the volume contractions are observed for all traditional porous metallic materials, while the volume expansion is found for the porous FeAl material, which must result from the different pore formation mechanisms. For example, the pores formed in the traditional porous metallic materials fabricated by powder metallurgy are due to the physics stowing of metallic powders. On the other hand, the pore formation mechanism of porous FeAl materials is believed to be the Kirkendall effect and consequent reactions [2]. For this reason, different pore formation mechanisms result in different pore morphologies, and, in turn, different permeabilities. Tortuosity factor is just the important parameter to describe the pore morphology.
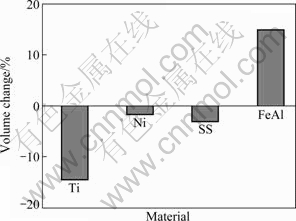
Fig. 2 Volume changes of different porous materials after sintering
3.2 Darcy law and tortuosity factor
Tortuosity here refers to the ratio of the true length to the apparent length (vertical extent) of a pore. A straight pore has a tortuosity factor of 1.0 whereas a tortuous pore has a tortuosity factor greater than 1.0 [8].
Tortuosity factor varies with structural parameters, such as open porosity and pore size of porous materials [12]. Therefore, to explore tortuosity factors for different samples, these samples should have the same open porosity and pore size. By adjusting fabrication conditions, we obtained the porous stainless steel and porous FeAl with the same open porosity of 30% and pore size of 2 μm.
Tortuosity factor can be used to estimate permeability [12]. Most studies show that the higher the permeability, the smaller the tortuosity factor [12]. The permeability (K) of porous material can be expressed by the Darcy law [13].
![]() (2)
(2)
where μ is the Darcy’s permeability coefficient; η is the dynamic viscosity of the fluid; L is the thickness of the sample. In this equation, μ/η can be determined experimentally through the practical measurement of the relationship between K and L. In doing so, the permeabilities of porous FeAl and porous stainless steel with the thickness varying between 1 and 5.5 mm were measured and results are shown in Fig. 3.
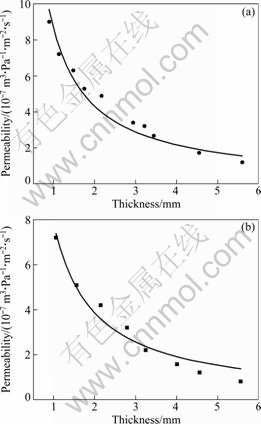
Fig. 3 Relationship between permeability and sample thicknesses: (a) Porous FeAl; (b) Porous stainless steel
Through the curve fitting, we obtain the following:
For porous FeAl,
![]() (3a)
(3a)
For porous stainless steel,
![]() . (3b)
. (3b)
As can be seen, the permeability of porous FeAl is always higher than that of porous stainless steel for any given thickness. Since they have the same open porosity and pore size, and since the higher the permeability, the smaller the tortuosity factor, the value of tortuosity factor of porous FeAl should be smaller than that of porous stainless steel.
3.3 Hagen-Poiseuille equation and tortuosity factor
The Hagen-Poiseuille equation [3] has been commonly used for describing the relationship among a system permeability and pore structural parameters including open porosity (θ), pore size (d), and pore morphology parameter, tortuosity factor (τ):
![]() (4)
(4)
Since the porous FeAl and porous stainless steel have the same θ and d, and η is a constant which is independent to the material, Eq. (4) can be written as:
![]() (5)
(5)
where λ is a constant related to the open porosity, pore size and dynamic viscosity of the fluid. As can be seen from Eq. (5), K is inversely proportional to τ. By comparing plots shown in Fig. 3, we can obtain that τFeAl<τSS.
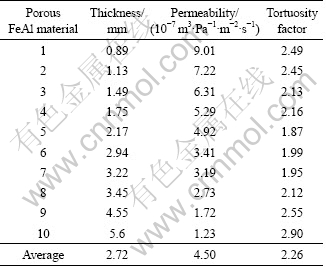
In our case, N2 was used as the fluid medium to measure the permeabilities, so that η=1.75×10-5 Pa·s [13]. Based on Eq. (4), the average tortuosity factors can be calculated as 2.26 for porous FeAl material (see Table 1) and 2.92 for porous stainless steel(see Table 2).
Table 1 Tortuosity factor calculated by Hagen-Poiseuille equation for porous FeAl material with porosity of 30% and the maximum pore size of 2 μm
3.4 Microstructure and pore formation mechanism for porous FeAl material and porous stainless steel
Figure 4 shows the microstructures of porous FeAl material and porous stainless steel.
Table 2 Tortuosity factor calculated by Hagen–Poiseuille equation for porous stainless steel material with porosity of 30% and the maximum pore size of 2 μm
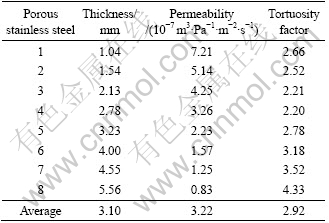
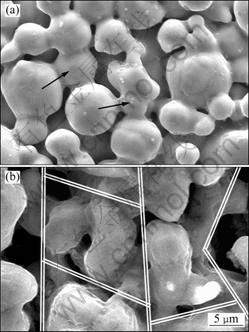
Fig. 4 Microstructures of pores in porous stainless steel (a) and porous FeAl (b)
As can be seen, the two porous materials have different pore morphologies, and the pore connectivity of porous FeAl is better than that of porous stainless steel. The pores in the porous stainless steel sample were formed by the evolvement of interparticle pores in alloy compact. The sintering procedure of porous stainless steels is typical solid sintering. During the sintering, the synergetic actions of the atomic surface diffusion and the surface tension lead to the atoms migrating to the contact points of alloy powders, which results in the enlargement of the contact points and the formation of sintering necks, which, in turn, results in the decrease of connecting pores. Therefore, the pore formation procedure of porous stainless steel is the shrinkage and closing of interparticle pores formed during the pressing procedure. The volume decrease of traditional porous metal materials after sintering can be then explained by the decrease of porosity during the sintering process. The pore structure of traditional porous metal materials can be adjusted by pressing pressure, raw powder size and sintering temperature. On the other hand, the pore formation in the porous FeAl materials is different from that in the porous stainless steel. Since the intrinsic diffusion coefficients of Fe (3.3×10-14 m2/s) and Al (1.1×10-12 m2/s) are great different [14], the net movement and consumption of Al must be balanced by the net vacancy flux, which will result in a large number of vacancies near the original positions of Al atoms[1]. The aggregation of excessive vacancies results in the pore formation to reduce the system Gibbs free energy [15]. For this reason, a large number of Kirkendall pores formed near or at the sites previously occupied by Al [16]. In addition, the consequent reactions between Fe and Al resulted in formation of a large amount of pores at the sites previously occupied by Al also, at the same time, the volume of the compacts swelled [17].
As evidenced in Fig. 4, different pore formation mechanisms result in different pore morphologies. In the case of porous stainless steels, the connecting pores can be easily cut off by sintering necks, as arrowed in Fig. 4(a). While in the case of porous FeAl material, since it is easy to form connecting pores during the fabrication process, these connecting pores possess strong connecting characteristic, as shown in Fig. 4(b).
Figure 5 shows the schematic diagram for the formation of pores in the cases of porous stainless steel and porous FeAl.
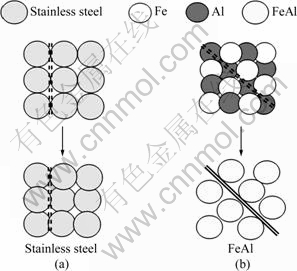
Fig. 5 Schematic diagram of pores evolving in porous stainless steel (a) and FeAl material (b)
Since the pore formation in porous stainless steel leads to the decrease of porosity and pore size in the compact, the pore connectivity in porous stainless steel fabricated by the powder metallurgy is expected to be worse. Therefore, the tortuosity factor of porous stainless steel should have a large value. On the other hand, the pore formation in the porous FeAl material leads to the increase of the porosity and pore size in the compact. Since pores form at the sites previously occupied by Al particles, the pore connectivity is expected to be better in the porous FeAl material. Consequently, the tortuosity factor of the porous FeAl material fabricated by reactive synthesis has a smaller value.
4 Conclusions
1) The tortuosity factor of porous FeAl material is smaller than that of porous stainless steel when their pore structures are identical. The average tortuosity factor is 2.26 for the porous FeAl material and 2.92 for the porous stainless steel, calculated by Hagen-Poiseuille equation.
2) The different pore formation procedures cause different natures of pore connectivities and tortuosity factors. The tortuosity factor in porous FeAl material has a smaller value, suggesting that the porous FeAl material has better filtration properties.
References
[1] GAO H Y, HE Y H, SHEN P Z, XU N P, ZOU J, JIANG Y, HUANG B Y, LIU C T. Porous FeAl intermetallics fabricated by elemental powder reactive synthsis [J]. Intermetallics, 2009, 17: 1041-1046.
[2] GAO Hai-yan , HE Yue-hui, SHEN Pei-zhi, XU Nan-ping, ZOU Jin, JIANG Yao, HUANG Bai-yun, LIU C T. Fabrication and pore structure characteristics of porous Fe-Al intermetallics [J]. Materials Science and Engineering of Powder Metallurgy, 2009, 14(4): 275-280. (in Chinese)
[3] BISWAS S, WINOTO S H. Prediction of pressure drop in non-woven filter media using a Hagen-Poiseuille model [J]. Tribology Transactions, 2000, 43(2): 251-256.
[4] REZAEE M R, MOTIEI H, KAZEMZADEH E. A new method to acquire m exponent and tortuosity factor for microscopically heterogeneous carbonates [J]. Journal of Petroleum Science and Engineering, 2007, 56: 241-249.
[5] ATTIA A M. Effects of petrophysical rock properties on tortuosity factor [J]. Journal of Petroleum Science and Engineering, 2005, 48: 185-198.
[6] ZALC J M, REYES S C, IGLESIA E. The effects of diffusion mechanism and void structure on transport rates and tortuosity factors in complex porous structures [J]. Chemical Engineering Science, 2004, 59: 2947-2960.
[7] MAO X, WANG S, SHIMAI S. Porous ceramics with tri-modal pores prepared by foaming and starch consolidation [J]. Ceramics International, 2008, 34(1): 107-112.
[8] RODRIGUEA R, ESTEVEZ M, VARGAS S, GONZALEZ M, SALAZAR R, PACHECO F. Synthesis and characterization of HAp-based porous materials [J]. Materials Letters, 2009, 63: 1558-1561.
[9] ALLAIRE-LEUNG S E, GUPTA S C, MONCRIEF J F. Water and solute movement in soil as influenced by macropore characteristics 2. Macropore tortuosity [J]. Journal of Contaminant Hydrology, 2000, 41: 303-315.
[10] ZHOU Wei-ping, XIANG Rong, WEI Fei, LUO Guo-hua. Quantitative description of the tortuosity of multi-walled carbon nanotubes by electron microscopy images [J]. Journal of Chinese Electron Microscopy Society, 2006, 25(2): 124-130. (in Chinese)
[11] FAN Rong-rong, LI Tao, ZHU Bing-chen, ZHU Ji-cheng. Engineering research of irregular shape catalysts with through-holes [J]. Journal of East China University of Science and Technology, 2002, 28(1): 1-4. (in Chinese)
[12] JAVID H A, ABDOLRAHIM J, MAHMOUD R P, MAJID N B. An approach to defining tortuosity and cementation factor in carbonate reservoir rocks [J]. Journal of Petroleum Science and Engineering, 2008, 60: 125-131.
[13] ISOBE T, KAMESHIMA Y, NAKAJIMA A, OKADA K, HOTTA Y. Gas permeability and mechanical properties of porous alumina ceramics with unidirectionally aligned pores [J]. Journal of the European Ceramic Society, 2007, 27: 53-59.
[14] SALAMON M, MEHRER H. Interdiffusion, Kirkendall effect, and Al self-diffusion in iron-aluminium alloys [J]. Inter J Mater Res Adv Tech, 2005, 96: 4-16.
[15] KANG H Z, HU C T. Swelling behavior in reactive sintering of Fe-Al mixtures [J]. Materials Chemistry and Physics, 2004, 88: 264-272.
[16] HE Y H, JIANG Y, XU N P, ZOU J, HUANG B Y, LIU C T, LIAW K. Fabrication of Ti-Al micro/nano-meter-sized porous alloys through the Kirkendall effect [J]. Advanced Materials, 2007, 19: 2102-2106.
[17] GEDEVANISHVILI S, DEEVI S C. Processing of iron aluminides by pressureless sintering through Fe+Al elemental route [J]. Materials Science and Engineering A, 2002, 325: 163-176.
反应合成法制备FeAl金属间化合物多孔材料的曲折因子
高海燕1,贺跃辉1,ZOU Jin2,徐南平3,C. T. LIU4
1. 中南大学 粉末冶金国家重点实验室,长沙 410083;2. 昆士兰大学 电镜中心,昆士兰 4072,澳大利亚;
3. 南京工业大学 膜科学技术研究所,南京 210009;4. 香港理工大学 机械工程系,香港
摘 要:以Fe、Al元素粉末为原料,采用反应合成方法,利用Kirkendall效应造孔制备的FeAl金属间化合物多孔材料是继陶瓷和金属多孔材料之后发展的一种新型多孔材料。曲折因子是表征多孔材料孔隙特征的重要参数。基于Darcy 原理 和 Hagen-Poiseuille 方程研究FeAl多孔材料的曲折因子。结果表明,FeAl多孔材料的曲折因子小于具有相同孔结构的不锈钢多孔材料的曲折因子。在本研究范围内,根据Hagen-Poiseuille 方程计算得知,FeAl多孔材料的曲折因子为2.26,而具有相同孔结构的不锈钢多孔材料的曲折因子为2.92。并由造孔机理探讨了FeAl多孔材料曲折因子较小的原因。
关键词:FeAl;金属间化合物;多孔材料;曲折因子
(Edited by YANG Hua)
Foundation item: Project (2009CB623406) supported by the National Basic Research Program of China; Projects (50825102, 50721003, 51071178) supported by the National Natural Science Foundation of China; Project (11JJ4036) supported by the Natural Science Foundation of Hunan Province, China; Project supported by the Central South University Free Exploring Project, China
Corresponding author: HE Yue-hui; Tel: +86-731-88836144; E-mail: yuehui@csu.edu.cn
DOI: 10.1016/S1003-6326(11)61446-5
Abstract: The tortuosity factor is the most critical parameter for the pore characteristic of porous materials. The tortuosity factor for porous FeAl intermetallics was studied based on the Darcy law and Hagen-Poiseuille equation. Porous stainless steel with the same pore structure parameter as porous FeAl was fabricated by powder metallurgy method for comparison. The results show that the tortuosity factor of porous FeAl intermetallics is smaller than that of porous stainless steel when their pore structure parameters are the same. The average tortuosity factor is 2.26 for the porous FeAl material and 2.92 for the porous stainless steel, calculated by Hagen-Poiseuille equation. The reason of the different tortuosity factors for porous FeAl and porous stainless steel was also explored through studying the pore formation mechanisms of the two types of porous materials.


