
Effect of Na2B4O7 on iron reduction in magnesium alloys AZ31 and AZ91
WU Guo-hua(吴国华), GAO Hong-tao(高洪涛), WANG Wei(王玮), DING Wen-jiang(丁文江)
School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200030, China
Received 15 July 2007; accepted 10 September 2007
Abstract:
Effect of Na2B4O7, a new iron reduction agent, on iron reduction in magnesium alloys AZ31 and AZ91 was studied. The iron contents in the magnesium alloy AZ31 and AZ91 reduce dramatically to less than 0.002% (mass fraction) with the increasing addition of Na2B4O7, and the corrosion resistance of the alloys was greatly improved. According to the thermodynamic analysis and the iron and boron distributions in different parts of the alloy melts, it can be inferred that the mechanism for iron reduction in magnesium alloys by Na2B4O7 processing is that boron atoms combine with iron atoms and settle down in the melting sludge. The XRD result confirms it.
Key words:
Na2B4O7; iron reduction; corrosion resistance; AZ31 magnesium alloy; AZ91 magnesium alloy;
1 Introduction
The applications of magnesium alloys are expanding with a high speed thanks to their low density and many other advantages[1-4]. However, the inherent quality of magnesium alloys has always been a knotty problem for researchers[5-6].
Nonmetallic inclusions and impurity elements have major detrimental effects on the inherent quality of magnesium alloys[7-10]. Some commercial fluxes can effectively remove nonmetallic inclusions from the magnesium alloys, but have no effect on the impurity elements namely iron[11]. It is known that iron severely decreases the corrosion resistance of the magnesium alloys[12]. And iron pickup is inevitable during melting, handling and machining of the magnesium alloys. Addition of manganese is a traditional method to reduce iron in the magnesium alloys[13]. However, the ratio of iron and manganese (a critical parameter for manganese processing) is hard to control and manganese is likely to segregate. In addition, HAITANI et al and TAMURA et al[14-15] believe that manganese disturbs the grain refinement of magnesium alloys. Therefore, we try to reduce the iron content in magnesium alloy by using purification flux containing some iron reduction agents. After large number of experiments we have found that Na2B4O7 can dramatically reduce the iron content in the magnesium alloys.
2 Experimental
Most popular wrought magnesium alloy AZ31 with initial iron content of 0.027% and casting magnesium alloy AZ91 with initial iron content of 0.032% were adopted in the experiments. The experimental alloys were refined by a mixture of Na2B4O7 and MgCl2, KCl, NaCl at a designed temperature. These chlorides act as solvent and have no iron reduction effect. After being stirred for 15 min, the melt was held for 600, 1 800 and 3 000 s. Then samples at the top, the center and the bottom of the melt were got for chemical analysis by an inductively coupled plasma spectrum machine (ICP, IRIS Advantage 1000).
The corrosion rates of the experimental alloys AZ31 and AZ91 by Na2B4O7 processing were determined by measuring the mass loss of the corrosion specimens exposed 5 d to ASTM D 1384-87 solution (148 mg/L Na2SO4, 138 mg/L NaHCO3 and 165 mg/L NaCl, pH 8.2)[16] maintained at room temperature, without stirring.
The melting sludge in the crucible after Na2B4O7 processing was studied by X-ray diffraction(XRD, D/MaxⅢ A-12KW-Cu detector, 40 kV voltage, 1(?)/min scanning rate) analysis. The database used for the phase identification of the X-ray diffractogram was JCPDS- International Centre for Diffraction Data.
3 Results
Fig.1 shows the iron content of the experimental alloy AZ31 melt by Na2B4O7 processing with different processing temperatures and holding times. After 0.6% addition of Na2B4O7 processing the iron content decreases to less than 0.0015%. Regardless of processing temperature and holding time, the iron content decreases rapidly with increasing the Na2B4O7 addition. For any fixed Na2B4O7 addition, the iron content can be reduced to a larger extent at higher processing temperature. This can be ascribed to that Na2B4O7 can react with iron to form some iron containing particles more effectively at higher temperature.
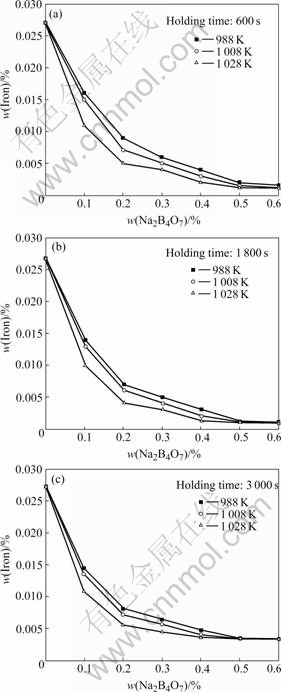
Fig.1 Relations between iron content in AZ31 alloy and Na2B4O7 additions at different holding times: (a) 600 s; (b) 1 800 s; (c) 3 000 s
Fig.2 shows the iron content of the experimental alloy AZ91 melt by Na2B4O7 processing with different processing temperatures and holding times. After 0.6% addition of Na2B4O7 processing the iron content decreases to less than 0.002%. The results are similar to those of AZ31 alloy before.
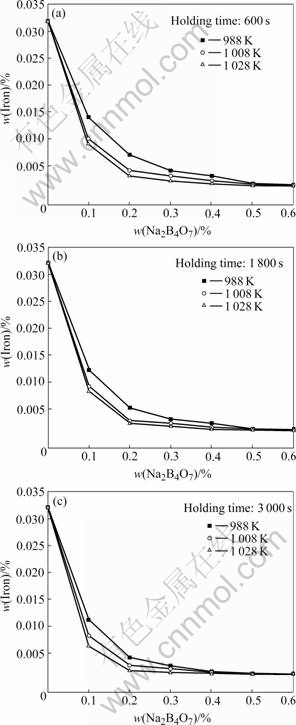
Fig.2 Relations between iron content in AZ91 alloy and Na2B4O7 additions at different holding times: (a) 600 s; (b) 1 800 s; (c) 3 000 s
Therefore, Na2B4O7 is confirmed to be a promising iron reduction agent for AZ series alloys.
Table 1 lists the corrosion rates of the experimental alloy AZ31 by Na2B4O7 processing. It can be seen that the corrosion rate decreases significantly from 17.2 mg/(cm2?d) to 0.8 mg/(cm2?d) with Na2B4O7 addition from 0 to 0.6%. Fig.3 (left) and (right) are the corroded surface photographs of the experimental alloy AZ31 without Na2B4O7 and with 0.6% Na2B4O7 processing respectively. The similar results for AZ91 alloy are shown as Table 2 and Fig.4. They indicate that Na2B4O7 can improve the corrosion resistance of the magnesium alloys, which is attributed to the effect of Na2B4O7 on iron reduction in magnesium alloys.
Table 1 Corrosion rates of experimental alloy AZ31 by Na2B4O7 processing
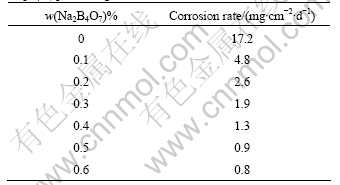
Table 2 Corrosion rates of experimental alloys AZ91 by Na2B4O7 processing
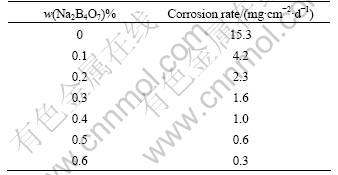
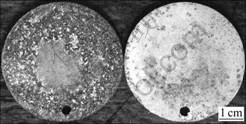
Fig.3 Corroded surface photographs of AZ31 alloy without Na2B4O7 (left) and with 0.6% Na2B4O7 (right) processing

Fig.4 Corroded surface photographs of AZ91 alloy without Na2B4O7 (left) and with 0.6% Na2B4O7 (right) processing
4 Discussion
We tried to analyze the reactions that occur in the chemically complicated alloy melt via thermodynamic calculations. The following reaction can be considered in magnesium melt.
(Na2B4O7)+6[Mg]+4[Fe]→6(MgO)+(N2O)+4(FeB) (1)
The change of reaction Gibbs free energy is
![]() (2)
(2)
Here, the parenthesis and the square brackets in the equation mean that the substance exists in the flux and the Mg melt respectively. ?GΘ is the change of Gibbs free energy of the reaction at 298.15 K and 100 kPa, and a(MgO), a(FeB), a(Na2B4O7), a(Na2O), a(Mg) and a(Fe) are the activities of MgO, FeB, Na2B4O7, Na2O, Mg and Fe in the melt respectively.
Unfortunately, some thermodynamic data are not easy to obtain because complicated reactions take place in the magnesium melt. We will try to carry out further work on it later. Now we make some reasonable hypotheses. MgO, FeB, Na2B4O7 and Na2O are assumed to be pure substance in the melt-flux system, i.e. a(MgO), a(FeB), a(Na2B4O7) and a(Na2O) are equal to be 1 respectively. And αMg and αFe are approximately replaced by their mole fractions for simplification. That is, for AZ31 alloy,
![]() (3)
(3)
![]() (4)
(4)
?G can be calculated to be
(5)
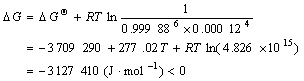
And for AZ91 alloy
![]() (6)
(6)
![]() (7)
(7)
?G can be calculated to be
(8)
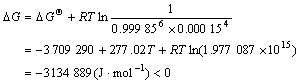
They indicate that reaction (1) can take place spontaneously thermodynamically. It is accordant with the experimental results before. That is to say, the reason for iron reduction in the magnesium alloys is that boron atoms combine with iron atoms and settle down.
In order to further study the iron reduction process, the iron and boron distributions in the top, the center, the bottom in the AZ31 melt with different holding times are measured and shown in Figs.5(a) and (b) respectively. Those for AZ91 melt are shown in Figs.6 (a) and (b) respectively.
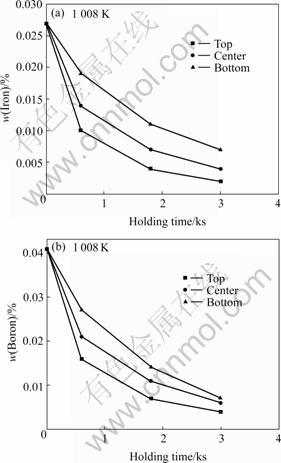
Fig.5 Iron (a) and boron (b) distributions in AZ31 melt by Na2B4O7 processing
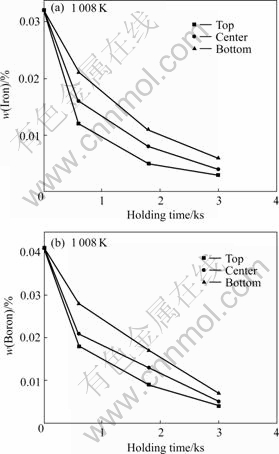
Fig.6 Iron (a) and boron (b) distributions in AZ91 melt by Na2B4O7 processing
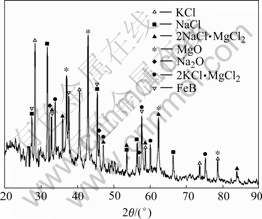
Fig.7 XRD pattern of melting sludge
It can be seen from Fig.5(a) that all of the iron contents in the top, the center and the bottom of the AZ31 melt decrease with the increasing holding time. Furthermore, the decreasing degree of the iron content in the top melt is the largest and that in the bottom melt is smallest. The boron distributions in the AZ31 melt shown as Fig.5(b) are of similar trend as the iron distributions.
Based on the iron and boron distributions in the magnesium melts we can infer that boron atoms combine with iron atoms and settle down in the crucible with the increasing holding time.
The XRD result of the melt sludge is shown in Fig.7. Though the phases in the melting sludge are complicated, we can find the iron-containing phase namely FeB from the XRD pattern. Therefore, it is confirmed that the formation of FeB is the main reason for the iron reduction in the magnesium alloy.
5 Conclusions
1) Na2B4O7 can reduce the iron content in the AZ31
and AZ91 magnesium melts to a considerable low level as less than 0.002%. Thus the corrosion resistance of the magnesium alloys is improved greatly.
2) The mechanism for the iron reduction by Na2B4O7 processing is that the boron atoms combine with iron atoms and settle down in the melting sludge.
Acknowledgement
The present study is funded by the National Basic Research Program of China (973 Program) and National Key -technologies R & D Program.
References
[1] ZOU Hong-hui, ZENG Xiao-qin, ZAI Chun-quan, DING Wen-jiang. Development in strengthening and toughening of magnesium alloys [J]. Materials For Mechanical Engineering, 2004, 28(5): 1-3.
[2] MORDIKE B L, EBERT T. Magnesium properties-applications- potential [J]. Mater Sci Eng A, 2001, 302(1): 37-45.
[3] WU Guo-hua, FAN Yu, GAO Hong-tao, ZHAI Chun-quan, ZHU Yan-ping. The effect of Ca and RE on the microstructure, mechanical properties and corrosion behavior of AZ91D [J]. Mater Sci Eng A, 2005, 408(1/2): 255-263.
[4] FAN Yu, WU Guo-hua, ZHAI Chun-quan. Effect of strontium on mechanical properties and corrosion resistance of AZ91D [J]. Mater Sci Forum, 2007, 546/549: 567-570.
[5] FAN Yu, WU Guo-hua, GAO Hong-tao, ZHAI Chun-quan. Current state and development of research on the corrosion of magnesium alloys [J]. Foundry Technology, 2004, 25(12): 941-944.
[6] GAO Hong-tao, WU Guo-hua, DING Wen-jiang, ZHU Yan-ping. Purifying effect of new flux on magnesium alloy [J]. Trans Nonferrous Met Soc China, 2004, 14(3): 530-536.
[7] PER B, KETIL P, SIGRID G. The impact of metal cleanliness in mechanical properties of die cast magnesium AM50 [J]. Magnesium Alloys and Their Applications, 2000: 739-745.
[8] TADASHI H, YOSUKE T, TETSUICHI M. Solubility of iron in pure magnesium and cast structure of Mg-Fe alloy [J]. Mater Sci Forum, 2003, 419-422.
[9] WU Guo-hua, KANG Seung-hun, YOU Bong-sun, YIM Chang-dong, SU Jang-rang. Effects of non-flux purification on the microstructure and mechanical properties of AZ31+xCa Mg alloy [J]. Mater Sci Forum, 2007, 546/549: 217-220.
[10] GAO Hong-tao, WU Guo-hua, DING Wen-jiang, ZHU Yan-ping. Study on Fe reduction in AZ91 melt by B2O3 [J]. Mater Sci Eng A, 2004, 368(1/2): 311-317.
[11] ZHAI Chun-quan, DING Wen-jiang, XU Xiao-ping. Development of new type hazardless fluxes used in the melting of Mg-alloys [J]. Special Casting and Nonferrous Alloys, 1997, 4: 48-50. (in Chinese)
[12] INOUE M, IWAI M, MATUZAWA K. Effect of impurities on corrosion behavior of pure magnesium in salt water environment [J]. Jpn Inst Light Met, 1998, 48(6): 257-262.
[13] LUNDER O, AUNE T K, NISANCIOGLU K. Effect of Mn additions on the corrosion behavior of mould cast magnesium ASTM AZ91 [J]. Corrosion, 1987, 43(5): 291-295.
[14] HAITANI T, TAMURA Y, YANO E. Grain refining mechanism of high-purity Mg-9mass%Al alloy ingot and influence of Fe or Mn addition on cast grain size [J]. J Jpn Inst Light Met, 2001, 51(8): 403-408.
[15] TAMURA Y, MOTEGI T, KONO N. Effect of minor elements on grain size of Mg-9%Al alloy [J]. Mater Sci Forum, 2000, 350/35: 199-204.
[16] A.S.T.M.D1384-87. Annul Book of ASTM Standards ASTM [S]. Philadelphia, PA 19103, 1986.
Corresponding author: WU Guo-hua; Tel: +86-21-62932164; Fax: +86-21-62932113; E-mail: ghwu@sjtu.edu.cn
(Edited by PENG Chao-qun)


