真空冶金回收废旧锌锰电池的汞和镉试验研究
何德文1,刘蕾1,肖羽堂2,周欢年1
(1. 中南大学 冶金科学与工程学院环境工程系,湖南 长沙,410083
2. 南开大学 环境科学与工程学院,天津,300071)
摘要:采用真空冶金方法处理锌锰电池,研究分离回收汞(Hg)和镉(Cd)的工艺方法,考察真空度、温度、加热时间对2种金属回收率的影响。试验结果表明:在真空度低于91.99 kPa时,Hg和Cd的回收率较低,但当真空度为91.99~98.66 kPa时,2种金属的回收率显著上升,超过98.66 kPa 时,Hg和Cd的回收率几乎保持不变;且随着温度的增加和加热时间的延长,Hg和Cd的回收率也增加,但当温度达到一定值和加热时间超过2.5 h时,Hg和Cd的回收率接近95%的饱和值。
关键词:
中图分类号:X703 文献标志码:A 文章编号:1672-7207(2011)04-0893-04
Experimental research on recycling of Hg and Cd from
waste zinc-manganic batteries by vacuum metallurgy
HE De-wen1, LIU Lei1, XIAO Yu-tang2, ZHOU Huan-nian1
(1. School of Metallurgical Science & Engineering, Central South University, Changsha 410083, China;
2. School of Environmental Science and Engineering, Nankai University, Tianjing 300071, China)
Abstract: A vacuum metallurgical process for treating waste zinc-manganic batteries and recovering mercury (Hg) and cadmium (Cd) elements was studied. The effect of vacuum degree, temperature and heating time on Hg and Cd removal was investigated. The experimental results show that vacuum metallurgical process can effectively recover Hg / Cd in waste batteries and its recovering rate increases with the increase of temperature and heating time. When the vacuum degree is 91.99-98.66 kPa, the heating time is 2.5 h; when the temperatures are 350 ℃ for Hg and 725 ℃ for Cd, the recovering rate is up to 95%.
Key words: zinc-manganic battery; vacuum metallurgy; mercury; cadmium; recycling and utilization
我国是世界上干电池生产和消费大国。据资料统计,20世纪末我国干电池产量约150亿只,占世界总量的50%[1-5],其中使用量最大的是锌锰电池。废旧的锌锰电池中汞和镉是剧毒污染物,对环境的污染已引起公众、媒体和环境保护部门的普遍关注[6-10]。但废旧电池的汞和镉又是十分贵重的金属,目前对于锌锰电池的回收利用技术主要是湿法冶金和火法冶金[11-14]。采用传统的冶金技术回收锌锰电池的汞和镉,都存在工艺流程复杂、能耗高、原材料消耗大且易产生二次污染等缺陷,在实际利用中有待改进。真空冶金法是冶金领域的新技术,与传统冶金相比具有能耗和资源消耗少、金属回收率高、成本较低等优点,特别对于金属汞和镉的冶炼具有明显优势[15]。在此,本文作者采用真空冶金法回收废旧锌锰电池的Hg和Cd,研究真空度、温度和加热时间对汞和镉回收率的影响。
1 实验部分
金属在一定的温度下有固定的蒸汽压,可表示为:
![]()
式中:a,b,c和d均为常数;P为蒸汽压;T为温度。
可见,环境的气体压强对金属蒸发有显著影响,对于废电池中Hg和Cd,可在较低能耗条件下,通过真空冶金的方法回收有价金属汞和镉。
实验材料为5号圆柱形锌锰电池,一部分是生活废弃物,另一部分是新电池。为了便于试验效果的比较,统一将新电池放电后再进行试验。真空冶金回收废旧锌锰电池工艺流程如图1所示,整个系统主要由加热器、冷凝器、真空计组成。

图1 真空冶金回收锌锰电池工艺流程
Fig.1 Process chart of recycling zinc-manganic batteries by vacuum metallurgy
废弃的锌锰电池表面带有污物,特别是有机污物,在真空条件下,污物的蒸气易释放出来,影响真空设备的工作压力,因此,为了改善材料的真空性能,在真空冶炼前,需对废旧锌锰电池进行预处理。一般先将废旧锌锰电池用碱清洗后,再用酒精洗干净,晾干再放入加热器中。
试验考察不同真空度、温度和加热时间下废旧锌锰电池汞和镉的回收率。汞、镉的含量采用火焰原子吸收光谱法结合化学滴定的方法测定[16-17]。
2 实验结果与讨论
2.1 真空度对汞、镉回收的影响
由于汞的沸点比镉的低,因此,Hg和Cd分别在450 ℃和700 ℃下加热2.5 h。不同真空度下的汞和镉的回收率结果如图2所示。
由图2可知:在较低的真空度条件下,Hg和Cd的回收率较低,且随真空度增大而缓慢增大;但当真空度超过一定值时(91.99 kPa时),2种金属的回收率显著上升,且与真空度存在线形关系,当真空度达到98.66 kPa 时,Hg和Cd的挥发率达到极限。

图2 真空度对汞和镉回收的影响
Fig.2 Effect of vacuum degree on recycling mercury and cadmium
2.2 温度对汞、镉回收的影响
控制真空度为99.99 kPa、加热时间为2.5 h,不同温度下的汞、镉回收率如图3所示。
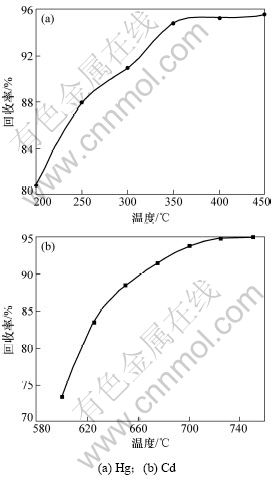
图3 温度对汞和镉回收率的影响
Fig.3 Effect of temperature on recycling mercury and cadmium
由图3可知:随着温度的增加,Hg和Cd回收率也增加;当温度在350 ℃以下时,Hg回收率与温度几乎呈线形关系;随后增加温度,回收率增长不显著,接近饱和。对于Cd,由于沸点比Hg的高,温度在600 ℃时,其回收率较低,随着温度的升高,回收率也显著升高,但超过700℃时,Cd的回收率也接近饱和。
2.3 加热时间对汞和镉回收的影响
保持真空度为99.99 kPa,Hg和Cd的加热温度分别为400 ℃和725 ℃时,加热时间对汞、镉回收率的影响如图4所示。
由图4可知,Hg和Cd的回收率随着加热时间的延长而增加,当达到2.5 h后,两者都不再显著提高。
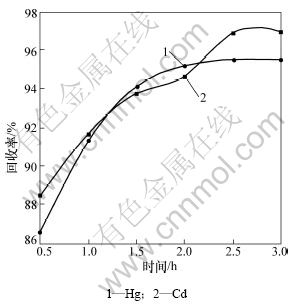
图4 加热时间对汞和镉回收的影响
Fig.4 Effect of heating time on recycling mercury and cadmium
3 结论
(1) 真空冶金工艺可有效处理锌锰废电池并分离回收其中Hg和Cd,不仅解决了汞和镉的污染问题,回收得到有价值的金属,而且具有回收成本低和利于工业化生产特点,具有很好的适用性。
(2)当真空度91.99~98.66 kPa时,Hg和Gd 2种金属的回收率显著上升;当真空度超过98.66 kPa 时,Hg和Cd的回收率几乎保持不变;且随着温度的增加和加热时间的延长Hg和Cd,回收率也增加,但温度达到一定值和加热时间超过2.5 h时,Hg和Cd的回收率接近95%,达到饱和。
参考文献:
[1] 李良, 邱克强, 陈启元. 对废电池处理的思考[J]. 电池, 2003, 33(2): 126-127.
LI Liang, QIU Ke-qiang, CHEN Qi-yuan. Thinking of waste battery treatment[J]. Battery, 2003, 33(2): 126-127.
[2] 马瑞新, 李国勋, 赵建民. 废干电池综合利用的研究[J]. 电池, 1999, 29(6): 275-278.
MA Rui-xin, LI Guo-xun, ZHAO Jian-min. Research on comprehensive using on waster battery[J]. Battery, 1999, 29(6): 275-278.
[3] 孔详华, 王晓峰. 旧镉镍电池湿法回收处理[J]. 电池, 2001, 31(2): 97-100.
KONG Xiang-hua, WANG Xiao-feng. Wet recovering and treatment waste on water battery containing Cd and Ni[J]. Battery, 2001, 31(2): 97-100.
[4] Xue Z H, Hua Z L, Yao N Y, et al. Separation and recover of nickel and cadmium from spent Ni/Cd storage batteries and their process waste[J]. Separation Science and Technology, 1992, 27(2): 213-221.
[5] 戴永年. 有色金属真空冶金[M]. 北京: 冶金工业出版社, 1998: 110-116.
DAI Yong-nian. Vacuum metallurgy on nonferrous metals[M]. Beijing: Metallurgy Industry Press, 1998: 110-116.
[6] Alguacil F J, Cobo A, Alonso M. Copper separation from nitrate/nitric acid media using Acorga M5640 extractant(Ⅱ): Solvent extraction study[J]. Chemical Engineering Journal, 2002, 85(2): 259-263.
[7] 刘彤宙, 李金惠, 聂永丰. 废锌锰电池真空蒸馏法去除汞的研究[J]. 环境污染治理技术与设备, 2006, 7(4): 114-119.
LIU Tong-zhou, LI Jin-hui, NIE Yong-feng. Study on mercury removal from waste Zn-Mn batteries by vacuum aided distillation technique[J]. Techniques and Equipment for Environmental Pollution Control, 2006, 7(4):114-119.
[8] 赵志星, 王存政, 吴宏斌. 废旧锌锰电池的去除和回收实验[J]. 环境卫生工程, 2005, 13(1): 14-16.
ZHAO Zhi-xing, WANG Cun-zheng, WU Hong-bin. Removing and recycle of mercury from waste zinc-manganese cell[J]. Environmental Sanitation Engineering, 2006, 13(1): 14-16.
[9] 李城芳. 汞在碱性锌锰电池中的作用及去除措施[J]. 电池工业, 2001, 6(4): 150-153.
LI Cheng-fang. Effects Function of mercury in alkaline Zn/MnO2 battery and removal measure[J]. Battery Industry, 2001, 6(4): 150-153.
[10] 卫碧文, 缪俊文, 龚治湘, 等. 分离富集-原子吸收光谱法测定锌锰电池中铅和镉[J]. 理化检验: 化学分册, 2007, 43(11): 925-929.
WEI Bi-wen, MIAO Jun-wen, GONG Zi-xiang, et al. FAAS determination of Pb and Cd in Zn/Mn battery with resin column enrichment[J]. PTCA (Part: Chem, Anal), 2007, 43(11): 925-929.
[11] 马明驹, 黄燕清, 潘伟, 等. 极谱法连续测定锌锰电池中铅和镉[J]. 电池, 2004, 34(3): 199-201.
MA Ming-ju, HUANG Yan-qing, PAN Wei, et al. Simultaneous determination of lead and cadmium in Zn/MnO2 battery by polarography[J]. Battery, 2004, 34(3): 199-201.
[12] 汝坤林, 秦以平. 锌锰电池中镉含量现状的调查[J]. 电池工业, 2000, 5(1): 37-40.
RU Kun-lin, QIN Yi-ping. Status of cadmium content in manganese dioxide-zinc battery[J]. Battery Industry, 2000, 5(1): 37-40.
[13] 瞿兆舟, 赵增立, 李海滨, 等. 回转窑热处理废锌锰电池试验研究[J]. 环境工程学报, 2008, 2(4): 542-548.
QU Zhao-zhou, ZHAO Zeng-li, LI Hai-bin, et al. Research on pyrolysis of spent alkaline Zinc Manganese dioxide batteries in a rotary kiln[J]. Chinese Journal of Environmental Engineering, 2008, 2(4): 542-548.
[14] Souza C C B, Tenorio J A S. Simultaneous recovery of zinc and manganese dioxide from household alkaline batteries through hydro-metallurgical processing[J]. Journal of Power Source, 2004, 136(1): 191-196.
[15] 朱建新, 李金惠, 聂永丰. 废旧电池回收利用的真空蒸馏装置, 中国, CN1363697A[P]. 2002-10-15.
ZHU Jian-xin, LI Jin-hui, NIE Yong-feng. A vacuum distillation equipment for recovering and using waste battery, China, CN1363697A[P]. 2002.
[16] ZHU Jian-xin, LI Jin-hui, NIE Yong-feng. Vaccum-aided recovery technology of spent Ni-Cd batteries[J]. Journal of Material Science and Technology, 2003(1): 55-58.
[17] Yasunori S, Yoshinori N, Yasuhiko Y. Disassembling and materials recovering processing of alkaline manganese dry batteries by vacuum-aided recycling systems technology[J]. Vaccum, 1999, 53(1/2): 101-104.
(编辑 赵俊)
收稿日期:2010-04-12;修回日期:2010-06-10
基金项目:国家自然科学基金资助项目(50274073);广东省教育部产学研结合项目(2009A090100016)
通信作者:何德文(1968-),男,湖南永州人,博士,副教授,从事污染控制技术的研究;电话:0731-88830875;E-mail:Hedewen@mail.csu.edu.cn


