Formation process of liquid in interface of Ti/Cu contact reaction couple
WU Ming-fang(吴铭方), YU Chun(余 春), YU Zhi-shui(于治水), LI ui-feng(李瑞峰)
(Department of Materials Science and Engineering, Jiangsu University of Science and Technology, Zhenjiang 212003, China)
Abstract:
By using the Ti/Cu contact reaction couples, the dissolution behavior of Ti and Cu in the eutectic reaction process was investigated under different conditions. The results show that the formation of eutectic liquid phase has a directional property, i.e. the eutectic liquid phase forms first at the Cu side and then spreads along the depth direction of Cu. The width of the eutectic liquid zone when Ti is placed on Cu is wider than that when Ti is placed under Cu. The shape of the upside liquid zone is wave-like. This phenomenon indicates that the formation process and spreading behavior in the upside are different from those in the underside, and there exists void effect in the Cu side of underside liquid zone, this will result in the delaying phenomenon of the contact reaction between Ti and Cu, and distinctly different shapes of the both liquid zones. The formation process of Ti/Cu eutectic liquid zone is similar to that of the traditional solid-state diffusion layer, and the relationship between the width of liquid zone and holding time obeys a square root law.
Key words:
Ti; Cu; contact reaction; dissolution; width of liquid zone CLC number: TG457.14;
Document code: A
1 INTRODUCTION
The method of using contact reaction of dissimilar metals to form an eutectic liquid to implement the joining of materials is called eutectic contact reactive brazing[1-3]. Compared with the traditional brazing or diffusion welding, the eutectic contact reactive brazing possesses properties such as low bonding temperature, little intermetallic compound in the joint, high remelting temperature, little distortion. The method has been found tremendous application potentials in engineering structural parts, especially in the field of joining materials which are difficult to join using general welding methods, such as Ni base alloys, Co base super heat resistant alloys[4], compound materials and non-metals[5]. Hence, this method is currently of wide interest.
As an interlayer for certain contact reactive brazing processes, Ti/Cu overlap-structure materials have a relatively wide use[6-9]. The application fields include joining metal to metal, metal to non-metal, and non-metal to non-metal. The main reasons are that as an active metal, Ti activates the surface of base materials when joining non-metals such as ceramic; and as an interlayer material, Cu can release stresses and prevent the joints from cracks. The braze-ability of dissimilar materials was studied using Ti/Cu overlap-structure materials as a contact reaction interlayer[6-9]. From the literatures, its not difficult to find that the studies of this field were mostly concentrated upon the thickness of Ti foil and Cu foil and the effects of process parameters on joint properties. However, there are few reports about the formation of the Ti/Cu eutectic liquid phase and the spread of liquid at the Ti/Cu interface. It is well known that when using Ti/Cu overlap-structure material as an interlayer, both the eutectic reaction products and the dissolution behaviour of Ti and Cu base metals affect the formation and properties of the joint greatly. In this paper, the effects of different brazing temperatures and holding time on Ti/Cu eutectic liquid behaviour are analyzed using Ti/Cu contact reaction couples. The results of this work can be used to assist in the accurate choice of Ti, Cu, or Ti/Cu as interlayer materials for conducting certain contact reactive brazing processes.
2 EXPERIMENTAL
The materials used in this work were pure Ti and pure Cu. The dimensions of specimens for joining were Ti 10mm×10mm×4mm and Cu 10mm×10mm×2mm. Before being placed into the vacuum furnace, the surface of each specimen was polished with 1# metallographic sand paper and burnished, and then were degreased in acetone with ultrasonic vibration followed by drying in a hot furnace. To compare the effects of different assemblage geometries of Ti and Cu on the eutectic reaction dissolution behaviour under the same process parameters, the specimens were assembled as a Ti/Cu/Ti sandwich structure. Furthermore, to avoid the liquid being extruded from the faying surfaces due to the self-weight of base metals, the specimens were assembled with an elastic equipment as shown in Fig.1.
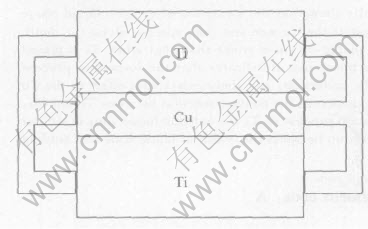
Fig.1 Sketch of specimen
Joining was performed in the temperature range of 880-940℃ and holding time of 1-180s in a high frequency induction-heating vacuum brazing furnace. To prevent the base metals from being oxidized, the vacuum degree was not higher than 5×10-3 Pa at high temperature. The heating rate was 1℃/s. After reaching the scheduled temperature and holding time, the specimens stopped heating and cooled to room temperature with the furnace power turned off.
For microstructural study, each bonded specimen was sectioned into two pieces. The width of the liquid zone and the interface morphologies were analyzed and compared by scanning electronic microscope(SEM, JSM-6300) equipped with energy disperse spectroscope(EDS, JXA-840A), the elements reaction and diffusion behaviour were studied with electron probe micro analysis(EPMA).
3 RESULTS AND DISCUSSION
3.1 Formation and spread mechanism of eutectic liquid phase
It can be seen from the Ti-Cu binary phase diagram that there are two eutectic reaction temperatures of 880℃ and 955℃, respectively. In this work, 880℃ was chosen as the reference temperature. To compare and analyze the effects of different temperatures on the behaviour of eutectic liquid, the brazing temperatures were 880℃, 900℃, 920℃, 940℃, respectively.
Fig.2 shows the SEM and EPMA analysis results of the contact reaction liquid zone from the experiment at 880℃ and 60s. It can be seen from the figure that the interfaces between the underside liquid zone and both the Ti and Cu base metals are planar, the width of the zone is about 460μm. But the interfaces between the upside liquid zone and the Ti and Cu base metals are wave-like, the width of the zone is about 850μm. The width of residual pure Cu is about 1050μm. Since the original width of copper is 2000μm, its not difficult to see that 950μm Cu base metal participated in the eutectic reaction and formed liquid phase under the condition of 880℃ and 60s. It illuminates that the reaction rate in Cu side is much quicker. But the width of the total consumed Ti in the reaction process is about 500μm. Compared with that of Cu base metal, the reaction rate is obviously slower. In addition, the results of EPMA test show that the quantities of Cu both in eutectic liquid zone and Cu base metal are almost equal, but the quantity of Ti in eutectic liquid zone is obviously less than that in Ti base metal. These further prove that the eutectic reaction rate in Cu side is quicker, and the dissolution quantity is bigger; but in Ti side, the eutectic reaction rate is slower, and the dissolution quantity is less. And the EPMA results show that the width of eutectic reaction liquid zone is much wider than the width of the solid-state diffusion zone of Ti and Cu base metals (the white zone between Ti/eutectic liquid phase zone and Cu/eutectic liquid phase zone). This is concerned with that the diffusion coefficient of elements in liquid is bigger than that of elements in solid.
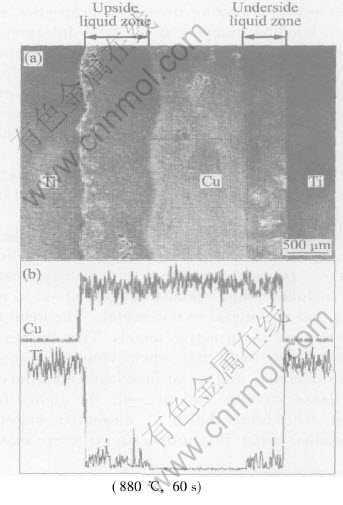
Fig.2 SEM and EPMA analysis results in reaction zone
Fig.3 shows the effects of temperature on the width and configuration of the liquid zones. The holding time is 60s, the brazing temperatures are 900℃ and 920℃, respectively. The configurations of liquid phase zones are similar to those shown in Fig.2, that is, the interface shapes between the underside liquid zone and both the Ti and Cu base metals are even, and the interface shapes between the upside liquid zone and the Ti and Cu base metals are wave-like. Because the diffusion rate of Ti in Cu is significantly higher than that of Cu in Ti(11.3cm2/s[10] and 0.57cm2/s[11] at 900℃, respectively); and from the Ti-Cu binary phase diagram[12], the saturation solution degree of Ti in Cu is about 7%(mole fraction) at 900-920℃, and that of Cu in Ti is about 45%(mole fraction), an acute eutectic reaction occurs between Ti and Cu, and the base metal at Cu side quickly dissolves, so the whole eutectic reaction has a direction with the enhanced temperature. The results of Ref.[13] that studied the dissolution behaviour of the base metals in Ti/Fe contact reaction proved the existence of this phenomenon, too.
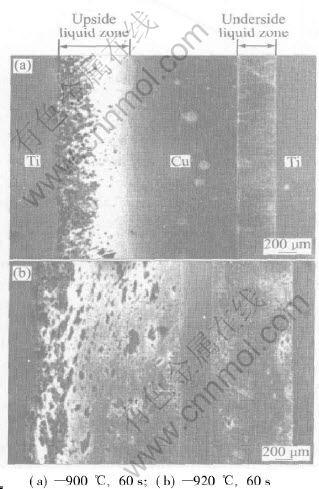
Fig.3 Effects of brazing temperature on width of eutectic reaction zone
From a dynamic viewpoint, for Ti/Cu contact reaction couples, the inter-diffusion ability of Ti atoms and Cu atoms increases with increasing temperature, and the diffusion driving force comes from the concentration gradient in the Ti/Cu interface. When the temperature reaches 880℃, and the eutectic components at the Ti/Cu interface are Ti 27%, Cu 73%(mole fraction), the liquid phase forms due to the eutectic reaction. This process can be called the first stage of the Ti/Cu eutectic reaction. After the formation of the liquid phase, the diffusion coefficients of Ti atoms and Cu atoms in liquid are much bigger than that in solid, so the base Cu and Ti dissolve quickly due to the eutectic reaction. Since the diffusion ability of Ti in Cu is stronger than that of Cu in Ti, and the saturation solution degree of Ti in Cu is smaller, the dissolution rate of Cu side exponentially increases. This process is called the second stage of Ti/Cu eutectic reaction.
Fig.2 and Fig.3 also show that the formation rate of the underside liquid phase zone is slower than that of the upside zone, and that the shapes are different. The interpretation of these phenomena are as follows. Ti/Cu is not a face-contact, but a multipoint-contact, therefore, the eutectic liquid phase disperses among the contact points, and almost concentrates on Cu side, as shown in Fig.4(a). Owing to the gravity and capillary effects, liquid in the underside liquid zone spreads along the surface of the base metals. Since the quantity of the liquid is relatively less, the spread of liquid results in the appearing of voids in the underside Cu base metal, as shown in Fig.4(b), and then the eutectic reaction at this zone stops. Only when the newly formed liquid fills in these voids, the eutectic reaction at this zone restarts, and the eutectic liquid phase advances into the Cu side in a planar way. The formation and spread mechanisms of the upside eutectic liquid phase zone are obviously different from those of the underside, the primary difference is that there are no voids in the Cu side. At the initial stage of the eutectic reaction, the contact points dissolve firstly in the Cu side and the eutectic reaction doesnt occur in the non-contact points until the eutectic liquid phase spreads from other zone to this one, this results in the formation of the upside liquid phase zone occurring in a wave-like manner, as shown in Fig.4(c). Since no voids appear, the eutectic reaction between Ti and Cu in this side is not delayed, and compared with the underside liquid phase, the formation rate of upside liquid phase obviously increases.
3.2 Effects of process parameters on width of eutectic liquid zone
Temperature is the most important parameter for contact eutectic reaction; a small change of temperature will result in a significant increase of
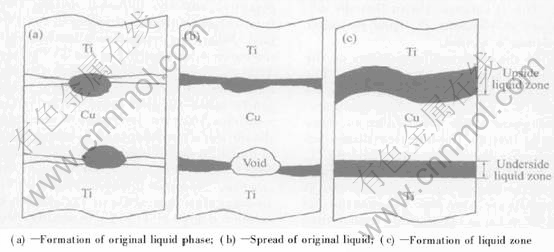
Fig.4 Formation processes of different eutectic reaction zones
reaction rate, which is proved by the phenomenon that the width of eutectic liquid zone increases greatly with increased temperature. As shown in Fig.3, when the temperature is 900℃ and the holding time is 60s, the widths of the upside and underside liquid zones are about 800μm and 400μm, respectively; at 920℃ and 60s, the widths are about 1700μm and 840μm, respectively. Its worth noting that under the condition of this work, when the brazing temperatures are 880℃ and 900℃, the counterpart widths of the upside and underside liquid zones change little. This can be obtained from Fig.2 and Fig.3. Maybe this is concerned with that there exists abundant of intermetallic compounds near Ti and Cu side during this temperature range, and the formation of intermetallic compounds blocks the inter-diffusion between Cu and Ti, which results in the synchronal decrease of dissolution rate.
Compared with the effect of temperature, the effects of holding time on the growth rate of eutectic liquid phase are subordinate. For Ti/Cu contact reaction couples, the influence rule is shown in Fig.5, where the temperature is 900℃, and the holding times are 1s, 30s, 60s, 120s. It can be seen from the figure that with the prolonging of holding time, the width of liquid zone increases gradually, and it is related linearly to t1/2. Tuah-Poku et al[14] studied the liquid phase formation process of Ag/Cu eutectic reaction, and found that the growth of liquid phase obeyed parabolic law; Taguchi et al[15] studied the compound growth rule of Ti/Cu diffusion reaction, and found that the growth of Ti-Cu compound obeyed the rule as the following formula:
d=kt1/2(1)
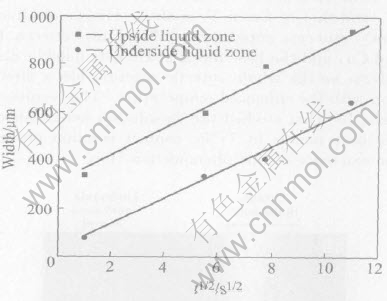
Fig.5 Relationship between width of liquid zone and holding time
where d is the width of compound layer, k is a constant, and t is the holding time.
Zhou et al[16] used a Ti/Cu/Ti overlap structure as an interlayer to join Si3N4 with partial transient liquid phase bonding, and found that the width of interface reaction layer also obeyed a parabolic law. The above experimental results show that the formation process of eutectic liquid phase layer is similar to that of solid-state diffusion layer, and the only difference is the formation rate.
Fig.5 also shows that the quantity of eutectic liquid phase in Ti/Cu contact reaction couples is related to the assemblage ways of Ti and Cu. When Cu is placed on Ti, not only the quantity of eutectic reaction is little, but also the spread of the liquid phase at the whole Ti/Cu interface has a delayed time; when Cu is placed under Ti, the width of eutectic liquid zone is bigger, the formation mechanism is related to the spread behavior of eutectic liquid phase at Ti/Cu contact interface.
The relation expressions between the width of liquid phase zone and t1/2 are got as follows:
W1=54t1/2+312(2)
W2=54t1/2+36(3)
where W1 and W2 represent the widths of upside liquid zone and underside liquid zone, respectively.
4 CONCLUSIONS
1) For Ti/Cu contact reaction couples, the formation of eutectic liquid has a direction, i.e. the eutectic liquid firstly forms at the Cu side, and then spreads along the depth direction of Cu.
2) The width of eutectic liquid zone when Ti is placed above Cu is wider than that when Ti is placed under Cu and the shape of the upside liquid zone is wave-like. The formation mechanism is related to the spreading and wetting behavior of eutectic liquid phase at the Ti/Cu contact interface.
3) The formation process of eutectic liquid zone is similar to that of a solid-state diffusion layer, the relationship between the width of liquid zone and holding time also obeys a square root law.
REFERENCES
[1]Lashko S V, Chulkov E I. Determination of process for the contact reactive brazing of contiguous tubular components in copper and stainless steel 1Kh18N9T [J]. Welding Production, 1975, 22(10): 46-48.
[2]Besednyi V A, Kornienko L V. Brazing of titanium alloys with iron-base solder [J]. Welding Production, 1976, 23(8): 46-48.
[3]Kruchinin V P, Metelkin I I. Contact reactive brazing of ceramics to metal [J]. Welding Production, 1972, 19(12): 68-73.
[4]Ikawa H, Nakao Y, Isai T. Research theoretical considerations on the metallurgical process in TLP bonding of nickel-base superalloys [J]. Transactions of the Japan Welding Society, 1979, 10(1): 24-29.
[5]Derby F B. Liquid phase bonding of siliconized silicon carbide [J]. Journal of Materials Science, 1995, 30(12): 6119-6135.
[6]Funamoto T, Wachi H, Kajiwara R, et al. Study on liquid phase diffusion welding of copper to austenitic stainless steel using Cu-Ti thin alloyed layer deposited by sputtering[J]. Japan Welding Society Journal, 1988, 6(2): 219-225.
[7]Wells R R. Microstructural control of thin-film diffusion-brazed titanium[J].Welding Research Supplement, 1976, 1: 20-27.
[8]Uzunov T D, Stojanov S P, Lambov S I. Contact-reactive welding of titanium via a copper layer [J]. Vacuum, 1999, 52: 365-368.
[9]Paulasto M, Cecone G, Peteves S D. Joining of silicon nitride vias transient liquid [J]. Scripta Materialia, 1997, 36(10): 1167-1173.
[10]Mazo N. The Metal Data [M]. Tokyo: the Japan Metal Society, 1974. 392.
[11]Sung Y L, Yoshiaki I, Osamu T, et al. Diffusion of copper and silver in β-titanium [J]. J Japan Inst Metals, 1990, 54: 502-508.
[12]Murray J L. The Cu-Ti system[J]. Bull Alloy Phase Diagram, 1983, 4: 81-95.
[13]WU M F, YU C, YU Z S, et al. A study of Ti/Fe contact reaction structures [J]. Materials Science and Technology, 2004, 20(5): 658-660.
[14]Tuah-Poku I, Dollar M, Massalski T B. A study of the transient liquid phase bonding process applied to a Ag/Cu/Ag sandwich joint [J]. Metallurgical Transactions A, 1998, 19A: 675-686.
[15]Taguchi O, Iijima Y, Hirano K. Reaction diffusion in the Cu-Ti system[J]. J Japan Inst Metals, 1990, 54(6): 619-629.
[16]ZHOU Fei, LI Zhi-zhang. Interfacial reaction and joint strength of Si3N4 partial transient liquid-phase bonded with Ti/Cu/Ti multi-interlayer [J]. The Chinese Journal of Nonferrous Metals, 2001, 11(2): 273-278.(in Chinese)
Foundation item: Project(JKLSDT-02-01) supported by the Open Fund of the Jiangsu Key Laboratory of Ship Designing Technology
Received date: 2004-07-07; Accepted date: 2004-11-10
Correspondence: WU Ming-fang, Associate Professor; Tel: +86-511-2910486; E-mail: yuchun1980@hotmail.com[


