文章编号:1004-0609(2007)04-0642-07
K2La2Ti3O10的制备和光催化产氢性能
杨亚辉,陈启元,尹周澜,李 洁
(中南大学 化学化工学院,长沙 410083)
摘 要:
通过聚合-配合方法和溶胶-凝胶方法,制备了具有层状钙钛矿结构复合氧化物K2La2Ti3O10光催化剂,采用X射线衍射(XRD)、紫外-可见漫反射光谱(DRS)、扫描电镜(SEM)等手段进行表征;以I-为电子给体,比较了制备方法对K2La2Ti3O10分解水产氢活性的影响。研究结果表明,溶胶-凝胶法制备的K2La2Ti3O10比聚合-配合法制备的K2La2Ti3O10光催化产氢活性要高出1倍左右,且制备条件友好,所得K2La2Ti3O10具有较好的单相性;获得了以I-为电子给体,溶胶-凝胶法制备的K2La2Ti3O10分解水的最佳实验条件:产氢的最佳pH值为11.5,RuO2的负载量为0.2%~0.3%。
关键词:
中图分类号:O 643.32 文献标识码:A
Preparation and photocatalytic activity of K2La2Ti3O10
YANG Ya-hui , CHEN Qi-yuan, YIN Zhou-lan, LI Jie
(College of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China)
Abstract: An ion-exchangeable layered perovskite type oxide, K2La2Ti3O10, was prepared by polymerized complex method and sol-gel method and characterized by XRD, DRS and SEM, et al. The influence of preparation method on the photocatalytic reactivity of K2La2Ti3O10 for hydrogen production was studied while I- was used as electron donor. The optimum condition for water splitting was investigated when K2La2Ti3O10 prepared by sol-gel method was used as photocatalyst. The results show that, the sol-gel method has superiority compared with the polymerized complex method. When K2La2Ti3O10 prepared by sol-gel method is used as photocatalyst for water splitting, the hydrogen production rate is twice of that prepared by polymerized complex. The preparation condition is kind and it is easy to get single phase K2La2Ti3O10 photocatalyst with sol-gel method. When K2La2Ti3O10 is used as photocatalyst with I- as electron donor, the optimum water splitting condition is obtained as follows: pH value is 11.5, the amount of loading RuO2 on K2La2Ti3O10 photocatalyst is 0.2%-0.3%.
Key words: sol-gel method; photocatalytic activity; hydrogen
1972年Honda[1]等通过TiO2光电转换将水分解为氢气,推动了光催化的发展。近几年来,利用半导体催化剂光解水制取氢气成为能源再生和存储的理想途径,采用光分解水制取氢气的研究[2-8]引起人们极大的兴趣。由于光催化材料的数量仍然有限,其催化活性偏低,因此,在利用半导体催化剂分解水制取氢气的研究过程中,制备新型高活性催化剂并拓展其对可见光的响应一直是人们的研究热点。层状钙钛矿结构的K2La2Ti3O10具有较好的光催化分解水的活性,现有的制备方法主要有聚合-配合法和高温固相法[9-15],而聚合-配合法制备的K2La2Ti3O10在紫外光辐射下分解纯水的产氢速率明显比高温固相法的高。K2La2Ti3O10是以Ti为主体的催化材料,有望通过其他软化学方法(如溶胶-凝胶法)优化制备条件,稳定材料性能,进一步克服高温固相法合成的催化材料实验重现性差、性能不稳定等不足。在此,本文作者通过聚合-络合方法和溶胶-凝胶方法制备具有层状钙钛矿结构的复合氧化物K2La2Ti3O10催化剂,并采用TG-DTA、XRD、DRS和SEM等分析手段对催化剂进行表征,以I-为电子给体[16-17],比较制备方法对K2La2Ti3O10分解水产氢活性的影响,研究溶胶-凝胶法制备的K2La2Ti3O10光催化分解水产氢的实验条件。
1 实验
采用日本理学D/max2250全自动转靶X射线衍射分析仪对K2La2Ti3O10催化剂进行XRD分析;采用美国Perkin-Elmer公司Lambda900紫外-可见-近红外分光光度计(带Labsphere积分球,BaSO4为参比标准白板)对催化剂进行DRS(漫反射光谱)分析;采用日本JEOL.LTD电子公司JSM—5600LV扫描电镜对催化剂进行SEM分析;采用美国Micromeritics Instrument Corporation公司ASAP2020比表面积孔径测定仪对催化剂进行BET分析;采用瑞士梅特勒-托力多公司TGA/SDTA851热重差热同步分析仪进行TG-DTA分析;采用SP-2305型气相色谱仪进行气相产物分析。
实验中使用的主要试剂有:钛酸丁酯C16H36O4Ti (化学纯,上海凌峰化学试剂有限公司生产);硝酸钾KNO3(分析纯,河南焦作市化工三厂生产);异丙醇(CH3)2CHOH(分析纯,仙桃市第一化工厂生产);La(NO3)3?nH2O(分析纯,上海国药集团化学试剂有限公司生产);KI(分析纯,焦作碱业集团化学试剂厂生产);KOH(分析纯,湖南师范大学化学试剂厂生产);柠檬酸(C6H8O7.H2O)(分析纯,上海山浦化工有限公司生产);乙二醇(CH2OH)2(分析纯,衡阳有机试剂厂生产);CH3OH(分析纯,湖南师范大学化学试剂厂生产);碳酸钾K2CO3(分析纯,江苏常熟化工厂生产);RuCl3?xH2O(分析纯,上海化学试剂一厂生产)。
1.2.1 聚合-配合法制备K2La2Ti3O10
将钛酸丁酯(C16H36O4Ti) 40 mmol溶解在16 mol CH3OH和0.8 mol乙二醇(CH2OH)2混合液中,在搅拌状态下加入柠檬酸(C6H8O7.H2O) 0.2 mol,溶解至透明后加入53.4 mmol K2CO3,搅拌到K2CO3完全溶解。然后加入26.6 mmol La(NO3)3?nH2O,在50 ℃搅拌几分钟得到透明溶液。将所得溶液加热至130 ℃,加速其聚合,除去过量的CH3OH、(CH2OH)2和H2O。在加热过程中溶液粘度逐渐变大,最终得到褐色凝胶。将凝胶在500 ℃烧结5 h得到前驱体。将前驱体充分研磨,再在一定温度的空气中烧结4 h,冷却、研磨,即可得到K2La2Ti3O10粉末材料[11]。
在本实验研究中,对所制备的催化剂负载一定量的RuO2[18-19]。称取K2La2Ti3O10粉体,加入到一定量的RuCl3溶液中,在磁力搅拌器上加热搅拌至水分完全挥发,然后充分研磨得到微细粉末,再在500 ℃的空气气氛中烧结5 h,即得到RuO2负载的K2La2Ti3O10催化剂,用于光催化分解水的实验。
光催化反应装置示意图见图1。
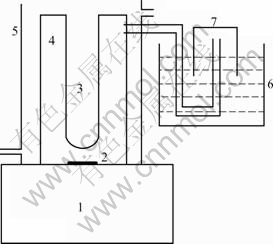
图1 光催化反应装置示意图
Fig.1 Device for photocatalytic water splitting: 1—Magnetic stirrer; 2—Magnetic bar; 3—250 W high-pressure Hg lamp; 4—Gas-closed inner irradiation cell; 5—Cooling jacket; 6—Water channel; 7—Gas collector
1.2.2 溶胶-凝胶法制备K2La2Ti3O10
称取0.02 mol硝酸镧和0.04 mol硝酸钾溶于22 mL蒸馏水中得到A液;称取钛酸丁酯(C16H36O4Ti) 0.03 mol溶于80 mL异丙醇中得到B液。在剧烈搅拌下将A 液逐滴加到B液中,继续搅拌5~10 min,在空气中静置3~4 h使其老化析出水和异丙醇,置于红外灯下烘干、充分研磨,再在一定温度的空气气氛中烧结4 h后冷却研磨,即得K2La2Ti3O10粉末[20-21]。按照同样的负载方法对溶液-凝胶法制备的K2LaTi3O10粉末进行RuO2负载,然后进行光分解水催化活性的研究。
1.3 光催化分解水实验
将250 W高压汞灯(发射光波长为300~400 nm,光照强度为15 000 μW /cm2,平均光照度为750 000 lx) 置入自制的约600 mL光反应装置中。反应前将600 mL的蒸馏水煮沸30 min以除去反应体系中的氧气,待其冷却至室温后加入反应装置中,再加入一定量的K2La2Ti3O10催化剂和一定量的KI,调节溶液pH值。在反应过程中采用磁力搅拌器使催化剂保持悬浮。产生的气体用气相色谱分析,产生气体的体积通过排水集气法收集和测定。
2 结果与讨论
2.1 聚合-配合法制备的K2La2Ti3O10的催化活性
图2所示为聚合-配合法制备的K2La2Ti3O10前驱体在25~1 000 ℃的空气气氛下烧结的TG-DTA曲线。在300℃以前的质量损失应归因于水分的失去和有机物的挥发,在300~700 ℃质量损失归因于前驱体中有机物的分解。在DTA曲线上可以看到2个明显的放热峰,分别在580 ℃和700 ℃附近,可能是由前驱体中有机物的燃烧引起。图3所示为K2La2Ti3O10前驱体在不同温度烧结后的XRD谱。可以看出,在700 ℃左右开始有K2La2Ti3O10晶体的衍射峰出现。但在图2中没有看到明显的K2La2Ti3O10晶体生成的放热峰,该峰可能被前驱体中有机物燃烧的放热峰所掩盖。在750~1 000 ℃之间仍有质量损失现象发生,特别在950 ℃以上较为明显,说明K2La2Ti3O10晶体生成以后,K元素在高温下可能产生挥发。

图2 聚合-配合法制备的K2La2Ti3O10前驱体的DTA-TG曲线
Fig.2 TG-DTA curves of K2LaTi3O10 precursor prepared by polymerized complex method
图3所示为聚合-配合法制备的K2La2Ti3O10前驱体在不同温度烧结后的XRD谱。可以看出,K2La2Ti3O10前驱体为无定型物质,其XRD衍射峰低而宽;在700 ℃烧结时,非晶相完全消失形成KLa2Ti3O9.5晶体,但是没有K2La2Ti3O10特征峰出现;在800 ℃时,K2La2Ti3O10 的特征峰仍然不很明显,此时烧结产物为KLa2Ti3O9.5 和K2La2Ti3O10的混合相;在900 ℃以上烧结时,KLa2Ti3O9.5完全消失生成完整的K2La2Ti3O10晶体。而且在900 ℃以上烧结时,K2La2Ti3O10 的衍射峰基本相同,只是随着烧结温度的升高,衍射峰的强度有所降低,可能是晶体生成以后K+的挥发造成的。
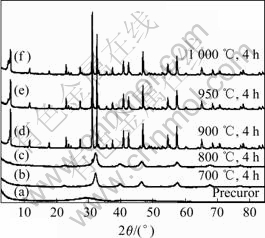
图3 聚合-配合法制备的K2La2Ti3O10前驱体在不同温度烧结后的XRD谱
Fig.3 XRD patterns of K2La2Ti3O10 precursor prepared by polymerized complex method and calcined at different temperatures
图4所示为经不同温度烧结后,聚合-配合法制备的K2La2Ti3O10光催化产氢量与反应时间的关系。从表1和图4都可以看出,在950 ℃烧结后,聚合-配合法制备的K2La2Ti3O10具有最佳的光催化产氢活性,12 h的产氢量为890 μmol? L-1。

图4 在不同温度烧结后聚合-配合法制备的K2La2Ti3O10光催化产氢量与反应时间的关系
Fig.4 Relationship between hydrogen content and reaction time of K2La2Ti3O10 photocatalyst prepared by polymerized complex method and calcined at different temperatures
从表1可以看到,随着烧结温度的升高,催化剂的颗粒有增大的趋势,经950 ℃烧结制备的K2La2Ti3O10具有较强的光催化分解水产氢活性。
表1 聚合-配合法制备的K2La2Ti3O10催化剂的产氢速率和BET比表面积
Table 1 The rate of hydrogen evolution and BET specific area of K2La2Ti3O10 photocatalyst prepared by polymerized complex method

2.2 溶胶-凝胶法制备K2La2Ti3O10的催化活性
图5所示为溶胶-凝胶法制备K2La2Ti3O10前驱体在25~1 000 ℃空气气氛下烧结的TG-DTA曲线。可见:在300 ℃以前的质量损失应归因于水分的失去和异丙醇的挥发,在300~450 ℃之间的质量损失归因于醇的分解和分解产物的挥发;在450~500 ℃有1个较大的质量损失,是KNO3的分解和挥发引起的;在500 ℃后的质量损失应该归因于La(NO3)3的逐步分解;在650 ℃和900 ℃附近的放热峰对应于K2La2Ti3O10晶体生成的放热峰,在900 ℃以上仍有质量损失现象发生。

图5 溶胶-凝胶法制备的K2La2Ti3O10前驱体的DTA-TG曲线
Fig.5 TG-DTA curves of K2La2Ti3O10 precursor prepared by sol-gel method
图6所示为溶胶-凝胶法制备K2La2Ti3O10前驱体在不同温度烧结后的XRD谱。可以看出,在800 ℃烧结后,生成的晶体不完整,此时所形成的产物为K2La2Ti3O10和La2TiO5的混合相;在900℃生成完整的K2La2Ti3O10晶体;当温度继续升高时,XRD谱基本一致。对比图3和图6可以看出,聚合-配合法制备的K2La2Ti3O10随着烧结温度的升高出现衍射峰减弱的现象,但溶胶-凝胶法制备的K2La2Ti3O10无类似现象,而且粉末具有较好的单相性。

图6 溶胶-凝胶法制备的K2La2Ti3O10前驱体在不同温度烧结后的XRD谱
Fig.6 XRD patterns of K2La2Ti3O10 precursor prepared by sol-gel method calcined at different temperatures
图7所示为在不同温度烧结后,溶胶-凝胶法制备的K2La2Ti3O10光催化产氢量与反应时间的关系。可以看出,溶胶-凝胶法制备的前驱体在950 ℃烧结得到的K2La2Ti3O10具有最佳的光催化产氢活性,12 h的产氢量为1 853 μmol?L-1,而在900和1 000 ℃烧结得到的K2La2Ti3O10具有相近的产氢活性。
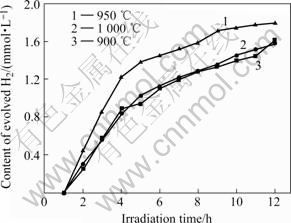
图7 在不同温度烧结溶胶-凝胶法制备的K2La2Ti3O10光催化产氢量与反应时间的关系
Fig.7 Relationship between hydrogen content and reaction time of K2La2Ti3O10 photocatalyst prepared by sol-gel method and calcined at different temperatures
但从表2可以看到,1 000 ℃烧结得到的K2La2Ti3O10具有最小的表面积,说明光催化活性的差异不是比表面积的差异所致,这一点与聚合-配合法所得产物的活性不同。因此,在950 ℃烧结后K2La2Ti3O10具有最佳的催化活性,这可能是因为所得K2La2Ti3O10结晶完整,颗粒之间界面光滑,避免了光生电子复合。
表2 溶胶-凝胶法制备K2La2Ti3O10催化剂产氢速率和催化剂的BET比表面积
Table 2 Rate of hydrogen evolution and BET specific area prepared by sol-gel method

2.3 溶胶-凝胶法和聚合-配合法制备的K2La2Ti3O10光催化活性的比较
不同的制备方法会影响催化剂粒径和分布的均匀性,因而影响光催化活性。一方面,制备方法不同,原料的来源和性质不同,催化剂的形成过程也不同,粒径和均匀性存在差异,粒子的表面性能就有所不同。采用聚合-配合法制备K2La2Ti3O10的过程中,柠檬酸稍过量,可以保证金属离子有足够的配合剂。但同时金属配合物具有稳定的五元环和六元环,因此,金属离子释放较慢,与溶胶-凝胶法制备K2La2Ti3O10相比,其离子间反应充分性不够。特别是由前驱体转为产品的过程中,后者使得产品结晶完整,具有较高的结晶度。
由表1和表2可以看出,溶胶-凝胶法制备的K2La2Ti3O10与聚合-配合法制备的相比,前者的光催化产氢活性提高了1倍左右。
图8所示为溶胶-凝胶法和聚合-配合法制备的K2La2Ti3O10的DRS比较结果。可见,在350 nm以下的紫外区,2种方法制备的K2La2Ti3O10吸光性能基本相同,但在380~450 nm的区域内,溶胶凝胶法制备的K2La2Ti3O10的吸光性能要比聚合-配合法制备的K2La2Ti3O10的吸光性能好。

图8 在950 ℃烧结后溶胶-凝胶法和聚合-配合法制备的K2La2Ti3O10的DRS比较图
Fig.8 Diffuse reflection spectra of K2La2Ti3O10 photocatalysts prepared by sol-gel method and polymerized complex method and calcined at 950 ℃
从图9所示的扫描电镜照片可以看出,溶胶-凝胶法制备的K2La2Ti3O10(图9(a))催化剂颗粒清晰可辩,结晶完整,而聚合-配合法制备的K2La2Ti3O10(图9(b))催化剂结晶度较低。

图9 溶胶-凝胶法和聚合-配合法制备的K2La2Ti3O10的SEM比较图
Fig.9 SEM photographs of K2La2Ti3O10 photocatalysts prepared by sol-gel method (a) and polymerized complex method (b) and calcined at 950 ℃
在碱性环境中(pH>9),K2La2Ti3O10催化剂受到光照产生光生电子和空穴,水溶液中的H+得到光生电子产生氢气,同时I-被光生空穴氧化为![]() ,其反应式如下[16-17]:
,其反应式如下[16-17]:
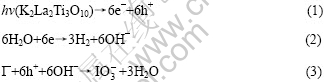
可见,反应溶液pH对催化剂的产氢活性有较大影响,为此,研究了溶液pH值对K2La2Ti3O10光催化产氢活性的影响。
此外,![]() 经光照可以发生歧化反应重新生成I-,电子给体I-得到恢复。
经光照可以发生歧化反应重新生成I-,电子给体I-得到恢复。
![]()
图10所示为反应溶液pH值对K2La2Ti3O10光催化活性的影响。可以看出,以I-为电子给体时,K2La2Ti3O10分解水的产氢活性受pH值影响较大,合适的溶液酸碱环境有利于K2La2Ti3O10分解水产生氢气。从以I-为电子给体光催化分解水的机理可知,溶液pH值过小,不利于I-转化为![]() ,电子给体不能有效地消耗光生空穴(h+),从而分离光生电荷。溶液pH值过大,将抑制水的电离,并有可能降低K2La2Ti3O10光生电子的还原能力。因此,以I-为电子给体时,K2La2Ti3O10分解水的产氢的最佳pH值为11.5。
,电子给体不能有效地消耗光生空穴(h+),从而分离光生电荷。溶液pH值过大,将抑制水的电离,并有可能降低K2La2Ti3O10光生电子的还原能力。因此,以I-为电子给体时,K2La2Ti3O10分解水的产氢的最佳pH值为11.5。

图10 反应溶液pH值对K2La2Ti3O10光催化活性的影响
Fig.10 Influence of pH value on K2La2Ti3O10 photocatalytic reactivity of K2La2Ti3O10 for hydrogen production
将K2La2Ti3O10表面负载一定量的贵金属氧化物RuO2进行实验,RuO2在减小光生电荷由催化剂内部向表面转移过程中的同时,还可以降低H2在催化剂表面的过电势。贵金属负载后的半导体催化剂可以起到微光伏电池作用,使分解水产氧和产氢的半反应分别在贵金属和半导体催化剂表面进行,提高K2La2Ti3O10光催化分解水的活性[18-19]。
在本实验中,确定负载量分别为0.1%,0.2%,0.3%,0.4%,0.5%和0.6%,考察负载量对K2La2Ti3O10光催化活性的影响,实验结果如图11所示。可以看出,RuO2负载量对K2La2Ti3O10的光催化活性有较大的影响,RuO2的负载量为0.2%~0.3%时可以取得较好的光催化产氢效果。
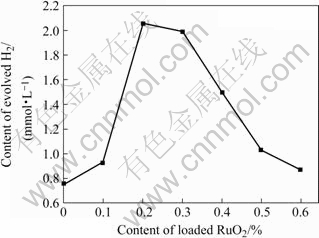
图11 RuO2的负载量对K2La2Ti3O10光催化活性的影响
Fig.11 Influence of amount of loaded RuO2 on photocatalytic reactivity of K2La2Ti3O10 for hydrogen production
3 结论
1) 采用聚合-配合方法制备了K2La2Ti3O10,对不同烧结温度下所制备的K2La2Ti3O10进行检测及表征。通过光催化活性表征可知,在950 ℃烧结得到的K2La2Ti3O10具有较高的光催化分解水产氢活性。
2) 采用溶胶-凝胶方法制备了K2La2Ti3O10,对不同烧结温度下所制备的K2La2Ti3O10进行检测及表征。通过光催化活性表征可知,于950 ℃烧结得到的K2La2Ti3O10较高的光催化分解水产氢活性。采用溶胶-凝胶法制备的K2La2Ti3O10比采用聚合-配合制备的K2La2Ti3O10光催化产氢活性要高1倍左右,制备条件友好,且获得的K2La2Ti3O10具有较好的单相性。
3) 以I-为电子给体,K2La2Ti3O10分解水产氢的最佳pH值为11.5;以I-为电子给体,且RuO2的负载量为0.2%~0.3%时K2La2Ti3O10具有较好的光催化产氢效果。
REFERENCES
[1] Honda K, Fujishima A. Eectrochemical photolysis of water at a semiconductor electrode[J]. Nature, 1972(238): 37-38.
[2] Karakitson K E, Verykios X E. Effect of altervalent cation doping of TiO2 on its performance as a photocatalyst for water cleavage[J]. Journal of Physical Chemistry, 1993, 97(6): 1184-1189.
[3] Sayama K, Arakawa H. Effect of carbonate salt addition on the photocatalytic decomposition of liquid water over Pt-TiO2 catalyst[J]. Journal of Chemical Society: Faraday Transactions, 1997, 93(8): 1647-1654.
[4] Bamwenda G R, Uesigi T, Abe Y, et al. The photocatalytic oxidation of water to O2 over pure CeO2, WO3, and TiO2 using Fe3+ and Ce4+ as electron acceptors[J]. Applied Catalysis A: General, 2001, 205(1/2): 117-128.
[5] Bamwenda G R, Arakawa H. Cerium dioxide as a photocatalyst for water decomposition to O2 in the presence of ![]() and
and ![]() species[J]. Journal of Molecular Catalysis A: Chemical, 2000, 161(1/2): 105-113.
species[J]. Journal of Molecular Catalysis A: Chemical, 2000, 161(1/2): 105-113.
[6] Abe R, Sayama K, Arakawa H. Significant effect of iodide addition on water splitting into H2 and O2 over Pt loaded TiO2 photocatalyst: suppression of backward reaction[J]. Chemical Physics Letters, 2003, 371(3/4): 360-364.
[7] Zou Z G, Ye J H, Sayama K, et al. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst[J]. Nature, 2001, 414(6864): 625-627.
[8] Zou Z G, Ye J H, Arakawa H, et al. Photophysical and photocatalytic properties of InMO4(M=Nb5+,Ta5+) under visible light irradition[J]. Materials Research Bulletin, 2001, 36(7/8): 1185-1193.
[9] Takata T, Shinohara K, Tanaka A, et al. A highly active photocatalyst for overall water splitting with a hydrated layered perovskite structure[J]. Journal of Photochemistry and Photobiology A: Chemistry, 1997, 106(1/3): 45-49.
[10] Thaminimulla C T K, Takata T, Hara M, et al. Effect of chromium addition for photocatalytic overall water splitting on Ni-K2La2Ti3O10[J]. Journal of Catalysis, 2000, 196(2): 362-365.
[11] Ikeda S, Hara M, Kondo J N, et al. Preparation of K2La2Ti3O10 by polymerized complex method and photocatalytic decomposition of water[J]. Chemistry of Materials, 1998, 10(1): 72-77.
[12] 张莉莉,杨 娟,张维光, 等. 硬脂酸法制备超细K2La2Ti3O10及其酸交换性质研究[J]. 无机化学学报,2003, 19(11):1217-1221.
ZHANG Li-li, YANG Juan, ZHANG Wei-guang, et al. Preparation of ultrafine K2La2Ti3O10 by stearic acid method and study on its acid-exchanging property[J]. Chinese Journal of Inorganic Chemistry, 2003, 19(11):1217-1221.
[13] Tai Y W, Chen J S, Yang C C, et al. Preparationofnano-goldon K2La2Ti3O10 forproducinghydrogenfromphoto-catalyticwater splitting[J]. Catalyst Today, 2004, 97(2/3): 95-101.
[14] Tong Z W, Zhang G Z, Takagi S, et al. Preparation and characterization of transparentthinfilm of the layered perovskite, K2La2Ti3O10, intercalatedwithanionic porphyrin[J]. Chemistry Letters, 2005, 34(5):632-633.
[15] CUI Wen-quan, LIU Li, FENG Liang-rong, et al. Preparation of Pt/K2La2Ti3O10 and its photo-catalytic activity for hydrogen evolution from methanol water solution[J]. Science in China: Series B Chemistry, 2006, 49(2): 162-168.
[16] Abe R, Sayama K, Domen K, et al. A new type of water splitting system composed of two different TiO2 photocatalysts (anatase, rutile) and a ![]() /I- shuttle redox mediator[J]. Chemical Physics Letters, 2001, 344(3/4): 339-344.
/I- shuttle redox mediator[J]. Chemical Physics Letters, 2001, 344(3/4): 339-344.
[17] Abe R, Sayama K, Arakawa H. Significant effect of iodide addition on water splitting into H2 and O2 over Pt loaded TiO2 photocatalyst: suppression of backward reaction[J]. Chemical Physics Letters, 2003, 371(3/4): 360-364.
[18] 岳林海,徐铸德. 半导体的表面修饰与其光电化学应用[J]. 化学通报, 1998, 9: 28-31.
YUE Lin-hai, XU Zhu-de. Surfacial modification of semiconductor and its applications in photo electrochemistry[J]. Chemical Letters, 1998, 9: 28-31.
[19] Amy L L, LU Guang-quan, John T Y. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results[J]. Chemical Reviews, 1995, 95(3): 735-758.
[20] 陈贞亮,王政存,申承民, 等. 溶胶-凝胶法制备纳米钛酸锶[J]. 功能材料, 1999, 30(6): 633-635.
CHEN Zhen-liang, WAN Zheng-cun, SHEN Cheng-min, et al. Preparation of nanocrystalline strontium titanate by the sol-gel process[J]. Journal of Functional Materials, 1999, 30(6): 633-635.
[21] 李 坤,陈王丽华,蔡忠龙, 等. 钛酸锶钡溶胶制备研究[J]. 江苏石油化工学院学报, 2000, 12(1): 1-4.
LI Kun, Chan W L H, Choy C L, et al. Preparation and properties of (Sr0.12Ba0.18)TiO3 solution[J]. Journal of Jiangsu Institute of Petrochemaical Technology, 2000, 12(1): 1-4.
基金项目:国家“863”计划资助项目(2002AA327140)
收稿日期:2006-09-06;修订日期:2006-12-19
通讯作者:杨亚辉,博士,讲师;电话:0731-8877364;E-mail:yangyh@mail.csu.edu.cn
(编辑 陈灿华)


