文章编号:1004-0609(2009)05-0854-07
酸浸蚀处理对AZ91D镁合金室温熔盐镀铝的影响
钱红妹,李岩,凌国平
(浙江大学 材料与化学工程学院,杭州 310027)
摘 要:采用酸性AlCl3-EMIC室温熔盐对AZ91D镁合金表面电沉积制备铝镀层,研究不同酸浸蚀处理对镀层与基体结合力的影响。采用刻划撕扯法对结合力进行判断,利用金相显微镜(OM)、SEM、EDS、XRD和XPS对镀层及酸浸蚀表面形貌、物相组成、元素价态进行观察和分析,采用盐雾实验法对镀层耐蚀性进行测定,并对镀层形貌的形成机理进行讨论。结果表明:对镁合金表面进行机械预处理后进行电沉积,得到的镀层不致密,且与基体的结合较差;对镁合金进行HF浸蚀后,仅能在镁合金表面得到致密均匀的铝镀层;而以稀磷酸为浸蚀液能同时改善镀层的致密性与基体的结合性能,镀层经两次撕扯后未脱落,对基体能起到良好的保护作用。
关键词:
中图分类号:TG 146.2;TG 174 文献标识码:A
Influence of acid pickling on electrodeposition of aluminum on magnesium alloy in room temperature molten salts
QIAN Hong-mei, LI Yan, LING Guo-ping
(College of Materials Science and Chemical Engineering, Zhejiang University, Hangzhou 310027, China)
Abstract: The effects of different acid pickling processes on the adhesions between the AZ91D magnesium alloy substrate and aluminum coatings electrodeposited from the acid AlCl3-EMIC room temperature molten salts were studied. The adhesions were estimated by score-tearing test. The surface morphology, phase compositions and element valence of coatings/etched substrate were analyzed by OM, SEM, EDS, XRD and XPS. The corrosion resistance was tested by salt-spray. The formation mechanism of coating morphologies was also discussed. The results show that the aluminum coating is not dense and the adhesion between the coatings and substrate is not good after mechanical pretreatment on AZ91D substrate. The dense and uniform coatings can be electrodeposited on the AZ91D substrate after pretreated by hydrofluoric acid pickling. The density of coatings and the adhesion can be improved simultaneously by using diluted phosphoric acid as pickling solution, and thus, the coating provides good protection to the substrate.
Key words: AZ91D magnesium alloy; room temperature molten salts; aluminum; acid pickling
镁合金作为实际应用中最轻的金属结构材料,具有低密度、高比强度和比模量以及优异的阻尼减震性能等优点,同时由于镁的储量丰富,在航空工业、汽车工业、电子通讯等设备上具有广泛的应用前景[1]。但镁的标准电极电位为-2.37 V,低于其他的金属结构材料以及其中的第二相或杂质而呈阳极,导致镁合金的耐蚀性差,因而需要对其表面进行保护处理[2]。
近年来,在镁合金表面上进行涂铝层已成为研究热点,这是因为如下几个原因:1) 铝是镁合金中的常见合金元素,它的加入不会增加镁合金回收利用的成本;2) 提高铝镁合金中的铝含量可以显著提高镁合金的耐蚀性[3];3) 铝与镁形成的金属间化合物可以作为一种较好的耐蚀和耐磨层[4-5];4) 铝的氧化膜致密坚硬,且具备自修复性。目前,在镁合金表面镀铝的方法主要如下:铝粉包埋扩散[6]、膏剂扩散[7]、电弧喷 涂[8]、超音速喷涂[9]、冷喷涂[10]以及气相沉积[11]等。
另一方面,电沉积镀铝具有镀层厚度可控、精度高的特点。通常电镀铝只能在有机溶剂、高温无机熔盐中进行[12-13]。室温熔盐是一种环保型离子液体,不含水和氧气,镀覆过程中无析氢等副反应发生,因此,可在多种金属表面上室温条件下制备致密的铝镀 层[14]。镁合金在室温熔盐中呈惰性[15],可以用室温熔盐对镁进行转化膜处理[16],CHANG等[17]报道了镁合金表面的室温熔盐镀铝。然而,镁合金在空气中极易氧化,形成的氧化膜比较疏松,表面覆层的结合力较差。因此,酸浸蚀对镁合金基体与覆层结合的影响成为镁合金表面防护处理的关键[18-19]。
本文作者研究了镁合金酸浸蚀对室温熔盐铝镀层与基体间结合力的影响。
1 实验
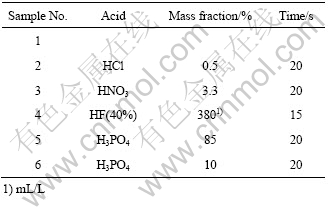
实验采用的基体材料为AZ91D压铸镁合金,尺寸10 mm×30 mm×1.5 mm。试样经过前处理后在真空手套箱内进行室温熔盐镀铝。前处理流程为:试 样→600#砂纸打磨→脱脂→酸浸蚀→丙酮超声清洗→吹干。实验中采用不同成分的酸浸蚀液(见表1)。电沉积溶液采用分析纯无水AlCl3与EMIC(1-甲基3-乙基咪唑氯化物),按摩尔比2?1配制成酸性室温熔盐体系,熔盐中主要含Al2Cl7。熔盐置于200 mL烧杯中,以镁合金为阴极,99.99%铝丝为阳极,采用直流电解,阴极电流密度2 A/dm2,沉积时间2 500 s。熔盐配制和电沉积都在氮气气氛下的真空手套箱内进行。沉积过程中,熔盐由磁力搅拌器磁力搅拌。
表1 酸浸蚀液的组成及酸洗时间
Table 1 Compositions of acid pickling solution and acid pickling time
采用光学显微镜(Lecia)、场发射扫描电子显微镜(SIRION-100)及所附的能谱分析仪(EDAX.Phoneix)分别对镀层进行了形貌观察和成分分析,采用X射线衍射仪(Riguka D/max-RA)分析镀层的表面物相组成,采用X射线光电子能谱仪(ESCLABMK Ⅱ型)分析试样表面的成分和元素价态,以5%NaCl的中性盐雾实验测试镀层的耐腐蚀性能,温度35 ℃,溶液pH值7.0,沉降量1~2 mL/h。由于镁合金上镀层结合力较差,因此,本研究中对镀层结合力采用划格撕扯法作定性检测。
2 结果与分析
在室温熔盐电沉积铝时,阴极和阳极表面发生以下电化学反应[20]:
对于阴极(试样):
![]()
对于阳极(铝丝):
![]()
结果试样附近的Al2Cl7-得到电子,在表面析出铝,而铝丝失去电子,并与溶液中的AlCl4-反应形成Al2Cl7-,使溶液中离子浓度基本保持恒定。
对试样1(未酸浸实验)进行室温熔盐镀铝,镀覆可以正常进行,镀铝后外观呈亚光的银白色。对试样进行了XRD检测,结果如图1所示。由图1可知,试样中有Al、Mg和少量Mg17Al12存在。Mg和Mg17Al12分别为AZ91D中的基体和第二相(见图2(a)),它们的存在说明Al镀层较薄。镀层的SEM形貌如图2(b)所示,铝镀层由尺寸约20 μm的球状颗粒组成,其中夹杂约4~5 μm的小颗粒。铝镀层没有覆盖整个表面,连续性和致密性较差;且铝颗粒以条状方式排列,与基体砂纸打磨的方向相一致。图2(c)所示为镀层的截面金相组织形貌,也显示出镀层的球状和不致密性。对镀层划格,一次撕扯后镀层与基体完全剥落,说明上述镁合金的前处理无法使铝镀层获得良好的结合性能。
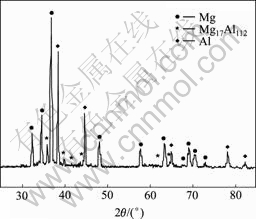
图1 镁合金室温熔盐镀铝层的XRD谱
Fig.1 XRD pattern of Al-coated Mg alloy in room temperature molten salts
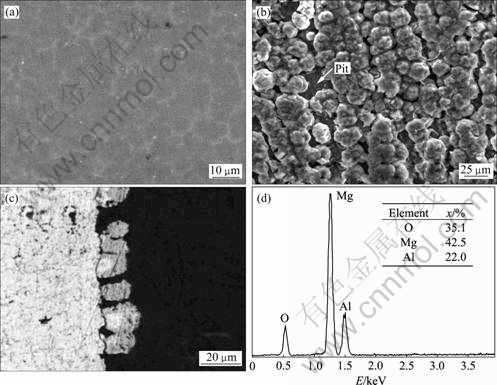
图2 机械预处理镁合金室温熔盐镀铝层的表面形貌及能谱结果
Fig.2 Micrographs and EDS pattern of Al-coated Mg alloy pretreated by mechine in room temperature molten salts: (a) SEM image of die-cast AZ91D Mg alloy substrate; (b) SEM image of Al-deposited sample; (c) Optical cross-section micrograph of Al-coated Mg alloy; (d) EDS pattern of hole in deposited layer
为了分析该镀层的欠致密性及与基体结合欠佳的原因,对镀层中凹坑部位进行了能谱成分分析,结果如图2(d)所示。由图2(d)可知,镀层中主要含O和Mg,分别达35.1%(摩尔分数)和42.5%,与MgO的成分相近。这可能是由于试样表面虽然经过充分打磨,但仍残留有MgO;也可能是试样在打磨过程或其后在空气中放置过程中,活性表面被迅速氧化所致。由于MgO膜的导电性差,电子无法通过疏松氧化膜被表面吸附的Al2Cl7-离子获得,因此,无法在这些位置上析出。
为彻底去除镁合金表面的氧化膜,对样品进行了砂纸打磨、脱脂、酸浸蚀、丙酮清洗,然后进行镀铝。但由于镁合金活性很高,采用表1所示的稀HNO3、稀HCl浸蚀后,得到的镀层外观有所改善,但结合力依然欠佳。这可能由于酸浸蚀后形成的活性表面又被迅速氧化的缘故。
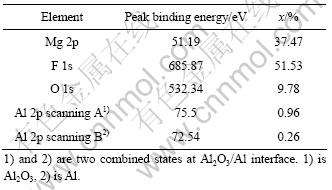
由于HF可以与镁合金形成MgF2 保护表面不被进一步氧化,因此,对脱脂后的试样采用高浓度HF溶液(380 mL/L)进行处理后电沉积铝,结果得到具有光亮银白色外观的镀层,铝层与镀层的形貌如图3所示。由图3(a)可知,镀层致密性好,除含少量10 μm左右的大颗粒外,镀层基本上由5 μm大小的具有明显晶体形貌的颗粒组成,颗粒与颗粒之间接触紧密。由表2可知,镀层的结合力欠佳。从图3(b)可见,镀层虽然连续致密,但与基体之间有一条明显的黑色界线。为了解释该现象,对HF溶液浸蚀后的试样表面进行了SEM观察和XPS分析,结果如图4和5所示。对比图4和图2(a)可以看出,镁合金经HF浸蚀后形貌发生明显的变化,原先Mg17Al12的位置上出现了腐蚀坑,而基体相平整光滑。由图5可知,表面存在Mg、F、Al和O元素,并以Mg和F为主(见表3)。分析高分辨谱图,可以推断,该膜由羟基氟化镁Mg(OH)2-xFx和微量Al2O3组成[21]。由此可做如下推测:首先Mg(OH)2-xFx为非金属,与铝之间没有金属键合;同时由于膜层光滑,与镀层之间没有机械锚合作用,因此,镀层与基体之间的结合力差。AZ91D浸入高浓度HF溶液中,反应进行约15 s基本停止,因此,可以认为所形成的MgF2膜很薄。这样,电镀时施加在基体和MgF2/溶液界面上的电场很高,能够产生隧道效应[22],使电子穿过MgF2膜被界面上吸附的Al2Cl7-离子捕获,从而使Al在MgF2膜上大量形核,并随电沉积的进行而长大,因此,镀层较致密。
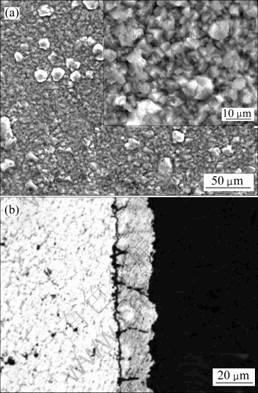
图3 浓HF酸浸蚀后镁合金室温熔盐镀铝层的形貌
Fig.3 Micrographs of Al-coated Mg alloy after dense HF acid pickling in room temperature molten salts: (a) SEM image of Al-deposited sample; (b) Optical cross-section micrograph of Al-coated Mg alloy
表2 不同酸浸蚀处理对镀层的影响
Table 2 Influence of different acid pickling on coatings
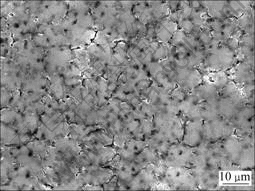
图4 浓HF处理后镁合金表面的SEM像
Fig.4 SEM image of Mg alloy after dense HF acid pickling
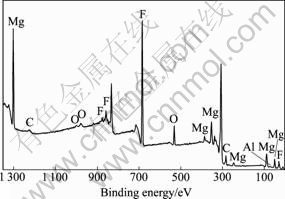
图5 浓HF酸浸蚀后镁合金表面的XPS谱
Fig.5 XPS patterns of surface of Mg alloy after dense HF acid pickling
表3 浓HF酸浸蚀后镁合金表面元素的结合能与含量
Table 3 Binding energy and molar fraction of element on surface of Mg alloy after dense HF acid pickling
为了同时获得镁合金上高致密性和结合力好的铝镀层,进一步对磷酸浸蚀处理进行了探讨。众所周知,磷化处理常用于金属表面上高分子涂层的前处理,因为磷酸盐膜与基体结合牢固,与高分子之间也具有良好的化学吸附或化学结合作用,其多孔形貌则有利于附着力的提高,且磷酸盐转化膜处理也被广泛应用于镁合金的耐蚀处理中。
首先采用85%(质量分数)浓磷酸进行浸蚀,其后室温熔盐镀铝。结果发现,镀层外观及结合力无明显改善。而采用10%稀磷酸溶液,经室温熔盐镀铝后,外观与浓HF溶液处理结果相似,并且镀层和基体的结合得到了显著的提高,镀层经划格并用胶带撕扯两次后,无任何损伤(表2中试样6)。镀层的SEM观察结果如图6(a)所示,可见铝的颗粒覆盖了整个基体表面,尺寸在15 μm左右。试样的XRD分析结果与图1所示的未经磷酸浸蚀试样相似,但铝峰的强度更高,镁基体峰的强度降低,由于两者铝镀层厚度相同,因此镁基体强度的降低与铝镀层完整的覆盖有关。对镀层与基体界面形貌进行观察,结果如图6(b)所示,由此可知,镀层与基体之间以锯齿状的方式结合。为了进一步分析结合力提高的原因,对稀磷酸浸蚀后试样6的表面进行了SEM观察,其结果如图7所示。由图7(a)可知,表面呈多孔形貌。由图7(b)的EDS分析可知,磷酸浸蚀后镁合金表面含Mg、Al、O和P,这可能是在表面形成了以Mg3(PO4)2为主的磷酸盐膜,这层膜的存在可以阻止镁合金的氧化。对铝镀层而言,磷酸盐膜的这种多孔形貌显然有利于提高镀层与基体之间的机械结合,但铝镀层与磷酸盐膜之间是否存在其他的结合机理还有待探讨。此外,致密铝镀层的获得,表明镁合金上形成的磷酸盐膜具有良好的导电性,因此,有必要深入研究电沉积初期铝在磷酸盐膜上的形核机理。
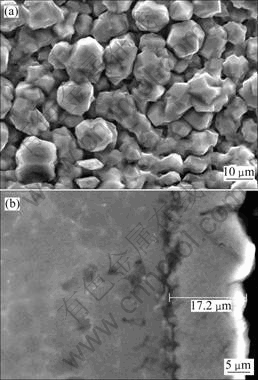
图6 稀磷酸浸蚀后镁合金室温熔盐镀铝层的表面与截面SEM像
Fig.6 SEM images of Al-coated Mg alloy with dilute phosphoric acid pickling: (a) Al-deposited sample; (b) Cross- section micrograph of Al-coated Mg alloy

图7 稀磷酸浸蚀后AZ91D表面的SEM像及EDS谱
Fig.7 SEM micrograph(a) of Mg alloy after dilute phosphoric acid pickling and EDS pattern(b) of surface
对稀磷酸浸蚀后镀铝的镁合金试样进行了耐腐蚀性能检测,试样经100 h中性盐雾实验后,镀层外观基本保持铝镀层的原色,未出现腐蚀现象。图8(a)所示为盐雾试验清洗后试样表面的SEM像。由图8(a)可见,镀层没有发生腐蚀开裂,仍保持良好的致密性,只是镀层铝颗粒的规则结晶棱边变成圆弧状,局部颗粒上有细微裂纹存在。镀层能谱面分析结果如图8(b)所示,表面主要含铝和氧,少量的镁来自基体。高的耐蚀性可能与铝在空气中形成致密的氧化膜及铝镀层的致密性有关。磷酸盐膜本身所具有的耐介质腐蚀能力也可能对镀层的耐蚀性起到有利的作用。
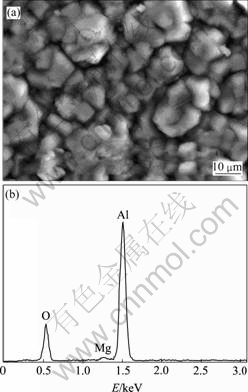
图8 盐雾腐蚀实验后镁合金室温熔盐铝镀层的SEM像及EDS谱
Fig.8 SEM image(a) of Mg alloy with dilute phosphatic acid pickling after 100 h spay salt test and EDS pattern(b) of this surface
3 结论
1) 机械前处理后镁合金表面进行室温熔盐镀铝,铝镀层不够致密,镀层与基体之间的结合性能较差。
2) 通过浓HF酸浸蚀可在镁合金上形成MgF2膜,从而改善铝镀层的外观,可得到致密的铝镀层,但不能提高镀层与基体的结合。
3) 通过稀磷酸浸蚀既可以改善铝镀层的致密性,也可以提高镀层与基体的结合力。多孔的磷酸盐膜阻止了镁合金氧化的同时,也有利于镀层与基体的机械结合。100 h盐雾实验表明,经稀磷酸浸蚀后,室温熔盐镀铝对AZ91D具有很好的保护作用。
REFERENCES
[1] 刘 正, 张 奎, 曾小勤. 镁基轻质合金理论基础及其应用[M]. 北京: 机械工业出版社, 2002: 1-34, 142-163.
LIU Zheng, ZHANG Kui, ZENG Xiao-qin. Theory and application of magnesium-base lightweight alloy[M]. Beijing: China Machine Press, 2002: 1-34, 142-163.
[2] GRAY J E, LUAN B. Protective coatings on magnesium and its alloys-a critical review[J]. Journal of Alloys and Compounds, 2002, 336(1/2): 88-113.
[3] SONG Guang-ling, ZHU Li-qun. Corrosion and protection of magnesium alloys: an overview of research undertaken by CAST[J]. Materials Science Forum, 2005, 488/489(6): 649-652.
[4] SONG Guang-ling. Recent progress in corrosion and protection of magnesium alloys[J]. Advanced Engineering Materials, 2005, 7(7): 563-586.
[5] HSIAO H Y, TSAI W T. Effect of heat treatment on anodization and electrochemical behavior of AZ91D magnesium alloy[J]. Journal of Materials Research, 2005, 20(10): 2763-2771.
[6] SHIGEMATSU I, NAKAMURA M, SIATOU N. Surface treatment of AZ91D magnesium alloy by aluminum diffusion coating[J]. J Mater Sci Lett, 2000, 19(6): 473-475.
[7] ZHU Li-qun, SONG Guang-ling. Improved corrosion resistance of AZ91D magnesium alloy by an aluminium-alloyed coating[J]. Surface and Coatings Technology, 2006, 200(8): 2834-2840.
[8] CHIU L H, CHEN C C, YANG C F. Improvement of corrosion properties in an aluminum-sprayed AZ31 magnesium alloy by a post hot pressing and anodizing treatment[J]. Surface and Coatings Technology, 2005, 199(2/3): 181-187.
[9] POKHMURSKA H, WIELAGE B, LAMPKE T, GRUND T, STUDENT M, CHERVINSKA N. Post-treatment of thermal spray coatings on magnesium[J]. Surface & Coatings Technology, 2008, 202(18): 4515-4524.
[10] 袁晓光, 刘彦学, 王怡嵩, 黄宏军. 镁合金表面冷喷涂铝合金的界面扩散行为[J]. 焊接学报, 2007, 28(11): 9-12, 16.
YUAN Xiao-guang, LIU Yan-xue, WANG Yi-song, HUANG Hong-jun. Influence of heat treatment on interfacial diffusion of Al alloy cold spraying coating on magnesium alloy[J]. Transactions of the China Welding Institution, 2007, 28(11): 9-12, 16.
[11] CHRISTOGLOU C, VOUDOURIS N, ANGEL-OPOULOS G N, PANT M, DANL W. Deposition of aluminum on magnesium by a CVD process[J]. Surface and Coatings Technology, 2004, 198(2/3): 149-155.
[12] WOLFGANG F A, DONALDSON S. Electroplating with aluminum[J]. Advanced Materials & Processes, 1996, 149(2): 33-35.
[13] 王吉会, 夏 扬, 王茂范. 无机熔盐镀铝层的制备与性能研究[J]. 兵器材料科学与工程, 2005, 29(6): 1-5.
WANG Ji-hui, XIA Yang, WANG Mao-fan. Preparation and properties of aluminum coating electroplated by inorganic molten salts[J]. Ordnance Material Science and Engineering, 2005, 29(6): 1-5.
[14] 凌国平, 逄请强. 室温熔盐电镀的研究进展[EB/OL]. 化学通报(网络版), 2000, c00035. http://www.hxtb.org/col.
LING Guo-ping, PANG Qing-qiang. The advance of electroplate in room-temperature molten salts[EB/OL]. Chemistry Online, 2000, c00035. http://www.hxtb.org/col.
[15] SHURANKOY A, EL ABEDIN S Z, ENDRES F. AFM-assisted investigation of the corrosion behavior of magnesium and AZ91 alloys in an ionic liquid with varying water content[J]. Aust J Chem, 2007, 60(1): 35-42.
[16] BIRBILIS N, HOWLETT P C, MACFARLANE D R, FORSYTH M. Exploring corrosion protection of Mg via ionic liquid pretreatment[J]. Surface and Coatings Technology, 2007, 201(8): 4496-4504.
[17] CHANG J K, CHEN S Y, TSAI W T, DENG M J, SUN I W. Electrodeposition of aluminum on magnesium alloy in aluminum chloride (AlCl3)-1-ethyl-3-methylimidazolium chloride (EMIC) ionic liquid and its corrosion behavior[J]. Electrochemistry Communications, 2007, 9(7): 1602-1606.
[18] ELSRNTRIECY H H, AZUMI K, KONNO H. Effect of surface pretreatment by acid pickling on the density of stannate conversion coatings formed on AZ91D magnesium alloy[J]. Surface and Coatings Technology, 2007, 201(3): 532-537.
[19] SUPPLIT R, KOCH T, SCHUBERT U. Evaluation of the anti-corrosive effect of acid pickling and sol-gel coating on magnesium AZ31 alloy[J]. Corrosion Science, 2007, 49(7): 3015-3023.
[20] ZHAO Y G, VANDER-NOOT T J. Electrodeposition of aluminium from non aqueous organic electrolyte systems and room temperature molten salts[J]. Electrochimica Acta, 1997, 42(1): 3-13.
[21] 丁培培. 镁合金化学镀镍影响因素及机理研究[D]. 杭州: 浙江大学, 2008: 84-89.
DING Pei-pei. Studies on influencing factors and mechanism of electroless nickel plating on magnesium alloy[D]. Hangzhou: Zhejiang University, 2008: 84-89.
[22] 孔庆升. 薄膜电子学[M]. 北京: 电子工业出版社, 1994: 35-39.
KONG Qing-sheng. Electron theory of thin films[M]. Beijing: Electronics Industry Press, 1994: 35-39.
收稿日期:2008-09-24;修订日期:2009-02-20
通讯作者:凌国平,教授,博士;电话:0571-87952648;E-mail: linggp@zju.edu.cn
(编辑 龙怀中)


