
Preparation of magnetic iron/mesoporous silica composite spheres and their use in protein immobilization
WANG Ping(王 平), ZHAO Jian-qing(赵建青), JIANG Zhi-jie(蒋智杰), LIU Yun-chun(刘运春),
LIU Shu-mei(刘述梅)
School of Materials Science and Engineering, South China University of Technology, Guangzhou 510640, China
Received 10 August 2009; accepted 15 September 2009
Abstract:
The iron/silica magnetic composite spheres were prepared by electrochemical method and reduced in hydrogen atmosphere at different temperatures. The morphology and structure of the composite were characterized by SEM, TEM and XRD. The iron/silica microspheres exhibit essential ferromagnetic behavior characterized by magnetometry. After being coated with silica in sodium silicate solution by acidifying technology, the surface of these magnetic composite spheres is with amino-silane coupling agent for their attachment to affinity ligands. Bovine serum albumin (BSA) was covalently immobilized onto the amino-silane modified magnetic silica supports by the glutaraldehyde method. The influence of pH, ionic strength as well as the initial protein concentration on BSA immobilization was studied. The results show that such amino-silane modified magnetic composite spheres are the effective supports for bioseparation and the maximum BSA immobilization capacity (up to 87.4 mg/g) is obtained in 0.1 mol/L phosphate buffer at pH 5.0.
Key words:
magnetic composite sphere; electrochemical synthesis; bovine serum albumin; immobilization; bioseparation;
1 Introduction
Magnetic supports have gained much attention recently due to their novel properties[1], which could have promising applications in immobilizing proteins, enzymes, and other bioactive agents in analytical biochemistry, medicine, and biotechnology[2-7]. The processes of isolation and separation of specific molecules are used in almost all areas of biosciences and biotechnology, and are the most documented and currently the most useful application of magnetic particles[8]. Magnetic separation is relatively rapid and easy, low cost and high efficient. For the present available magnetic supports used in bioseparation, low binding capacity and slow mass transfer kinetics are two limiting factors that restrict the applications of the supports to laboratory scale only[9]. One method to eliminate both limitations at a time is to provide a significant reduction in particle size with large surface area required, which may carry enough magnetite, and an appropriate surface functionality with high enough density of functional groups on supports for efficient coupling of affinity ligands [10]. Mesoporous silica (MS) spheres with high surface areas, 3D pore networks and pore sizes in the range of 2-50 nm are used as a matrix of synthesizing magnetic particles[11-12]. If one could combine the advantages of mesoporous silica and magnetic particles to fabricate a magnetic composite with high surface area and magnetic property, a novel magnetic carrier would be developed based on mesoporous material and high-capacity magnetic supports would be prepared for protein separation.
A great deal of research has been done to synthesize various magnetic particles as magnetic carriers in separation processes to overcome the inadequacies of separation techniques commonly used for bioseparation and bioanalysis[13-17]. LIU et al[18] reported the synthesis of magnetic silica nanospheres with the core-shell structure of magnetite (Fe3O4)/silica and their use for protein immobilization. SEN et al[19] described the template-assisted fabrication of magnetic mesoporous silica-magnetite nanocomposite and its potential for application in magnetic bioseparations. HERDT et al[20] proposed the bioconjugation of histidine-tagged enzymes and other proteins to the surface of composite “magnetomicelles” consisting of magnetic g-Fe2O3 nanoparticles encapsulated within cross-linked polystyrene-block-polyacrylate copolymer micelle shells. But the concept of using mesoporous silica spheres as porous media to prepare magnetic composite spheres as immobilisation matrices (supports; carriers) for protein bioseparation is very novel and effective and there remain unexplored frontiers from the viewpoints of practical applications and basic sciences. Magnetic composite spheres with high surface areas and high magnetism are being used in the biotechnology that allow high protein loadings and remain the activity of bioactive substances. In this work, we demonstrated the synthesis of magnetic nanoparticles within the pores of mesoporous silica through an electrochemical method. The obtained composite microspheres were thermally reduced in hydrogen atmosphere to produce iron/silica composites whose magnetic properties were evaluated. Then their surface was coated with silica in sodium silicate solution by acidifying technology. The surface of the above obtained composite spheres was with amino-silane coupling agent for their attachment to affinity ligands. Bovine serum albumin (BSA) was covalently immobilized onto the amino-silane modified magnetic silica supports by the glutaraldehyde method, as schematically outlined in Fig.1.
2 Experimental
2.1 Materials
Hexadecylamine was purchased from Fluka. Amino-silane coupling agent (AEAPS), BSA, glutaraldehyde and glycerol were obtained from Sinopharm Chemical Reagent Company. Ethyl silicate, isoprorylalcohol ammonia, ethanol and other reagents were of analytical grade. All solutions were prepared with doubly distilled water.
2.2 Preparation of MS spheres
Mesoporous silica spheres used were synthesized according to Ref.[21]. Typically, 1 g of n-hexadecylamine was dissolved in a mixture of 100 mL of isopropanol and 90 mL of H2O, and 1.4 mL NH3?H2O (28%) to form a homogenous solution. Afterwards, 12 mL of TEOS was added to the solution at once while stirring, then the mixture was left static overnight at room temperature, and the product, collected through filtration, was washed with deionized water. To remove the surfactants, the as-synthesized material was heated in air at 600 ℃ for 6 h. To facilitate the incorporation of magnetic nanoparticles, we adopt three ingredient salts with m(NaCl)?m(LiCl)?m(KNO3)=20?5?5 to enlarge the pore size of silica oxides. MS spheres were soaked in the salt solution for a period of time. Then the mixture was calcined in air at 300 ℃ for 2 h.
2.3 Synthesis of magnetic composite spheres
Magnetite nanoparticles were synthesized according to Ref.[22]. We took a low-cost and easy-available metal iron plate and a sheet of stainless steel as anode and cathode, respectively, to synthesize nanosized Fe3O4 particles at room temperature. After treating iron plate and stainless steel thin sheet, they were respectively used to be anode and cathode of electrolysis, and the space between them was 5 mm. Electrolytes were 0.02 mol/L Na2S2O3, the pH was then fixed at 10 with concentrated sodium hydroxide solution. After the electrolysis completing, the solution obtained from reaction firstly was concentrated. The residue was washed with deionized water. The obtained composite particles were reduced for 3 h at constant temperature in a hydrogen stream introduced into the reactor at a constant flow rate of 2 L/h. The samples were then cooled to room temperature under the hydrogen atmosphere. The reduction temperature was varied from 300 to 400 ℃ to modulate the magnetic properties of the composites. Thus, iron/silica composite spheres were prepared.
2.4 Synthesis of magnetic composite spheres
The surface of magnetic composite spheres was treated by amino-silane[18]. 2 g magnetic composite spheres prepared above, 2.5 mL H2O and 10 mL AEAPS were added into 250 mL methanol. The mixture was treated by ultrasound for 30 min, mixed with 150 mL glycerol and then transferred to a 500 mL three-necked flask equipped with a mechanical stirrer. The temperature was kept at 85-90 ℃ with rapid stirring for 3 h. The resulting nanospheres were washed with deionized water and methanol several times. These composite spheres were immersed in 5% (volume fraction) glutaraldehyde solution with 0.1 mol/L phosphate buffer, pH 7.4 for 6 h at room temperature and then washed with deionized water.
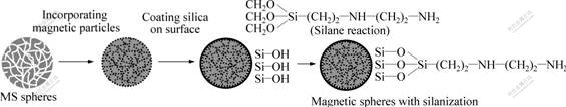
Fig.1 Illustration of procedures for preparing iron/silica composite microspheres with grafting AEAPS
In a typical experiment, an appropriate amount of BSA was dissolved in 5 mL of buffer solution of specified pH and 25 mg of glutaraldehyde activated magnetic supports was added. The mixture was incubated at 30 ℃ for 4 h. Then the magnetic supports were separated by permanent magnet. The amount of BSA immobilized onto magnetic supports was determined by measuring the initial and final concentrations of BSA from the absorbance at 280 nm of the supernatant using a calibration curve prepared previously. In order to remove perfectly a very small amount of wafting particles, all the separated supernatants were centrifuged before measuring.
2.5 Characterization techniques
Scanning electron microscopy (SEM) was taken on a Philips XL30 scanning electron microscope at 20 kV which was used to examine the particle size and shape and the effectiveness of the coating procedure. Wide-angle (0-80?, 40 kV, 200 mA) X-ray powder diffraction (XRD) data were recorded on a Rigaku D/max 2 550 VB/PC diffractometer using nickel-filter. Magnetic characterization of the samples was carried out in a vibrating sample magnetometer (VSM). The saturation magnetization (Ms), coercivity (Hc) and squareness (Mr/Ms, where Mr is the remanent magnetization) were obtained from the hysteresis loops for each sample recorded at 298 K after applying a saturating field of 3 T. The Ms values were evaluated by extrapolating to infinite field. The experimental results were obtained in the high field rang where the magnetization linearly decreases with 1/H. Protein concentrations were measured from the absorbance at 280 nm by a Shimadzu UV-2102PC spectrophotometer.
3 Results and discussion
3.1 Synthesis of iron/silica composite spheres
Fig.2 shows the SEM images of the iron/silica composite spheres obtained after reduction at different temperatures. As shown in Fig.2, a layer of iron nanocrystalline was observed on the surface of silica spheres. When the sample was reduced at 300 ℃, the amount of pure iron nanoparticles coated on the surface of MS spheres was relatively little. The amount of iron particles increases gradually with increasing reduction temperature. The grain volume distribution changes gradually from nonuniform modal to a nearly cubic grating. Otherwise, the surface of composite spheres has a uniform distribution of iron particles with increasing reduction temperatures to 400 ℃. The microstructure of the coatings consists of a large amount of iron particles surrounded by and incorporated into the mesoporous silica. This behavior indicates that the temperature is the important factor for the effect of reduction of iron nanoparticles. The X-ray diffraction patterns obtained after reduction at different temperatures are shown in Fig.3. As observed, the most intense peak corresponding to Fe is detected in all reduced samples along with the broad feature at 2θ between 20? and 30? due to amorphous silica and irrespective of treatment temperature. As expected, a narrowing α-Fe peak is observed under the condition of increasing the reduction temperature, indicating an increase of the crystalline size.
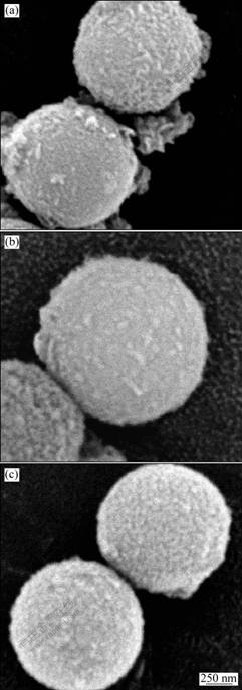
Fig.2 SEM images of iron/silica composite microspheres obtained via electrochemical approach and reduced at different temperatures under H2 atmosphere
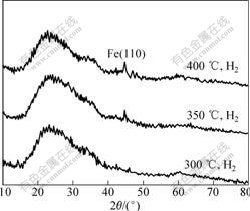
Fig.3 XRD patterns of iron/silica composite microspheres obtained via electrochemical approach and reduced at different temperatures under H2 atmosphere
3.2 Magnetic characterization of iron/silica composite spheres
Fig.4 shows that the magnetism curves of iron/silica composite spheres at different temperatures. As observed, the saturation magnetization of the sample reduced at 300 ℃ is much lower (1.1 A?m2/kg), taking into account the iron/Si atomic ratio of the sample and the Ms value of 220 A?m2/kg for iron[23]. This behavior indicates that an important part of the iron crystallites is oxidized, which can be at least in part due to an incomplete reduction in agreement with the increase of Ms (2.0 A?m2/kg) observed as increasing the reduction temperature up to 350 ℃. It should be noted that this Ms value still lower than the theoretical one could be increased to 2.9 A?m2/kg at a further temperature increase up to 400 ℃, suggesting that the remaining iron oxide might result from a partial re-oxidization of the iron crystallites in the air atmosphere rather than from an incomplete reduction.
The values observed from Fig.4 of Hc are 27 382, 31 521and 38 526 A/m respectively at different reduction temperatures of 300, 350 and 400 ℃. The changes are very little. These Hc values are similar to the theoretical values expected for non-interacting single-domain spherical iron particles oriented at random[24]. Finally, it should be noted that the Mr/Ms values measured for these samples (<0.3) are significantly lower than the theoretical ones expected for an assembly of spherical single-domain and non-interacting particles with cubic crystal anisotropy (three <100> easy axes) randomly oriented (0.83)[23]. The results of magnetic properties exhibit essential characteristics of ferromagnetic behavior approach to that of spherical iron/silica nanocomposites from core-shell particles reported by OCANA et al [25].

Fig.4 Magnetization curves measured at room temperature for iron/silica composite particles obtained at different reduction temperatures
3.3 Protein immobilization studies
The influence of different factors on the immobilization capacity (mg/g) of BSA onto the glutaraldehyde activated magnetic supports was studied. Fig.5 shows the effect of pH on BSA immobilization with the initial BSA concentration 3 mg/mL in the 0.1 mol/L phosphate buffer at room temperature. When the pH value (varied from 3 to 9) is 5.0, which is at around the isoelectric point (pI) of BSA (pI=4.7), immobilization capacity of BSA reaches the biggest value, which is in agreement with previous report[18]. There is a neutral charge at the isoelectric point, whereas at pH different from the pI the molecules are charged and repelled each other. As a result, the neutral environment benefits the BSA adsorption on magnetic/silica support surface and repulsion force between homogeneous charges significantly reduces the BSA immobilization amount. From Fig.5, we can see that the amount of BSA immobilization at alkaline

Fig.5 Effect of pH on BSA immobilization (initial BSA concentration of 3 mg/mL at room temperature)
solution is higher than that in acid solution. We conclude that some charges exist under basic condition, which can lessen the repulsion force and increase the amount of BSA immobilization.
Fig.6 shows the change of the amount of BSA immobilization at different BSA concentrations in 0.1 mol/L phosphate buffer with pH 5.0. As shown in Fig.6, the amount of BSA immobilized increases with increasing BSA concentration, but reaches a plateau value at 3 mg/mL of BSA concentration. The maximum immobilization capacity is about 87.4 mg/g at an initial BSA concentration of 3.5 mg/mL. After eluting again and again, there is few unattached BSA on the magnetic composite supports and adsorbed BSA is covalently immobilized onto the glutaraldehyde activated magnetic composite supports.
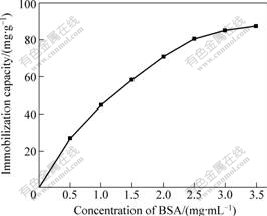
Fig.6 Effect of initial BSA concentration on immobilization (pH 5.0, at room temperature)
Fig.7 shows the effect of the ionic strength on the BSA immobilization in sodium chloride solutions (from 0 to 1 mol/L) with the initial BSA concentration of 3 mg/mL in 0.1 mol/L phosphate buffer with pH 5.0. The result shows that the amount of the BSA immobilized increases with the increase of NaCl concentration from 0 to 0.6 mol/L. In the converse, the further increase in the ionic strength above 0.6 mol/L up to 1.0 mol/L NaCl causes the decrease of the amount of BSA immobilization. The maximum immobilization capacity is realized at pH=pI, where the protein has a neutral charge and minimum solubility. In the conclusion, ion strength does not greatly affect the BSA immobilization at pH 5.0.
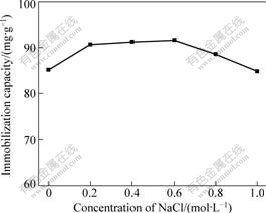
Fig.7 Effect of NaCl concentration on BSA immobilization (pH 5.0, initial BSA concentration of 3 mg/mL at room temperature)
4 Conclusions
1) A novel method for the preparation of highly functionalized magnetic supports for bioaffinity separation was demonstrated, which have the average diameter of about 1.2 μm in diameter and exhibit magnetic characteristics.
2) The magnetic supports have the advantage of high mechanical rigidity and biocompatibility. The results show that synthesized magnetic composite spheres with high surface areas allow high protein loading (87.4 mg/g) and remain the activity of bioactive substances.
3) The synthesized magnetic composite spheres provide the potential of this technique in the field of affinity separation, especially for antibody purification, immunoassay, and cell isolation.
References
[1] Doyle P S, Bibette J, Bancaud A, Viovy J L. Self-assembled magnetic matrices for DNA separation in lab on a chip [J]. Science, 2002, 295: 2237-2237.
[2] Haukanes B I, Kvam C. Application of magnetic beads in bioassays [J]. Biotechnology, 1993, 11: 60-63.
[3] Safarik I, Safarikova M. Use of magnetic techniques for the isolution of cells [J]. J Chromatogr B, 1999, 722: 33-53.
[4] Meldrum F C, Heywood B R, Mann S. Magnetoferritin: In vitro synthesis of a novel magnetic protein [J]. Science, 1992, 257: 522-523.
[5] Tanaka T, Matsunaga T. Fully automated chemiluminescence immunoassay of insulin using antibody-protein A-bacterial magnetic particle complexes [J]. Anal Chem, 2000, 72: 3518-3522.
[6] Hong X, Li J, Wang M J, Xu J J, Guo W, Li J H, Bai Y B, Li T J. Fabrication of magnetic luminescent nanocomposites by a layer-by-layer self-assembly approach [J]. Chem Mater, 2004, 16(21): 4022-4027.
[7] Kiselev M V, Gladilin A K, Melik-Nubarov N S, Sveshnikov P G, Miethe P, Levashov A V. Determination of cyclosporin A in 20% ethanol by a magnetic beads-based immunofluorescence assay [J]. Analytical Biochemistry, 1999, 269: 393-398.
[8] H?feli U, Schütt W, Teller J, Zborowski M. Scientific and clinical applications of magnetic carriers [M]. New York: Plenum Press, 1997.
[9] Xue B, Sun Y. Fabrication and characterization of a rigid magnetic matrix for protein adsorption [J]. J Chromatogr A, 2002, 947(2): 185-193.
[10] Liu X, Guan Y, Liu H, Ma Z, Yang Y, Wu X. Preparation and characterization of magnetic polymer nanospheres with high protein binding capacity [J]. J Magn Mater, 2005, 293: 111-118.
[11] Napolsky K S, Eliseev A A, Knotko A V, Lukahsin A V, Vertegel A A, Tretyakov Y D. Preparation of ordered magnetic iron nanowires in mesoporous silica matrix [J]. Mater Sci Eng, 2003, 23: 151-154.
[12] Ryoo R, Ko C H, Kim J M, Howe R. Preparation of nanosize Pt clusters using ion exchange inside mesoporous channel of MCM-41 [J]. Catal Lett, 1996, 37: 29-33.
[13] Smith J E, Wang L, Tan W T. Bioconjugated silica-coated nanoparticles for bioseparation and bioanalysis [J]. TrAC Trends Anal Chem, 2006, 25: 848-855.
[14] SAFARIK I, SAFARIKOVA M. Magnetic techniques for the isolation and purification of proteins and peptides [J]. Biomagn Res Technol, 2004, 2(1): 7-10.
[15] Liu C Z, Honda H, Ohshima A, Shinkai M, Kobayashi T. Development of chitosan-magnetite aggregates containing nitrosomonas europaea cells for nitrification enhancement [J]. J Biosci Bioeng, 2000, 89: 420-425.
[16] van Hee P, Hoeben M A, van der Lans R G J M, van der Wielen L A M. Strategy for selection of methods for separation of bioparticles from particle mixtures [J]. Biotechnol Bioeng, 2006, 94: 689-709.
[17] Horák D, Rittich B, ?panová A, Bene? M J. Magnetic microparticulate carriers with immobilized selective ligands in DNA diagnostics [J]. Polymer, 2005, 46: 1245-1255.
[18] Liu X, Xing J, Guan Y, Shan G, Liu H. Synthesis of amino-silane modified superpara-magnetic silica supports and their use for protein immobilization [J]. Colloid Surf A, 2004, 238: 127-131.
[19] Sen T, Sebastianelli A, Bruce I J. Meso-structured superparamagnetic nanospheres and nanotubes: Smart materials for bioseparations [J]. J Am Chem Soc, 2006, 128: 7130-7131.
[20] Herdt A R, Kim B S, Taton T A. Encapsulated magnetic nanoparticles as supports for proteins and recyclable biocatalyst [J]. Bioconjugate Chem, 2007, 18: 183-189.
[21] Grun M, Buchel C, Kumar D, Schumacher K, Bidlingmaier B, Unger K K. Rational design, tailored synthesis and characterization of ordered mesoporous silicas in the micron and submicron size range [J]. Stud Surf Sci Catal, 2000, 128: 155-165.
[22] Franger S, Berthet P, Berthon J. Electrochemical synthesis of Fe3O4 nanoparticles in alkaline aqueous solutions containing complexing agents [J]. J Solid State Electrochem, 2004, 8: 218-223.
[23] Cullity B D. Introduction to magnetic materials [M]. Massachusetts: Addison-Wesley Publishing Company, 1972.
[24] Zijlstra H. Permanent magnets theory [J]. Ferromagnetic Materials, 1982, 3: 37-105.
[25] Ocana M, Andres-Verges M, Pozas R, Serna C J. Spherical iron/silica nanocomposites from core-shell particles [J]. J Colloid Interface Sci, 2006, 355: 294-361.
(Edited by LI Xiang-qun)
Foundation item: Project(10676009) supported by the National Natural Science Foundation of China; Project(20080440750) supported by the Postdoctoral Science Foundation of China
Corresponding author: WANG Ping; Tel: +86-20-22236818; E-mail: wangping_2009@126.com


