
Dominance of Acidithiobacillus at ore surface of Zijinshan commercial low-grade copper bioleaching heap
LIU Xing-yu(刘兴宇), CHEN Bo-wei(陈勃伟), WEN Jian-kang(温建康)
National Engineering Laboratory of Biohydrometallurgy, General Research Institute for Nonferrous Metals,
Beijing 100088, China
Received 20 September 2008; accepted 5 November 2008
Abstract:
The microbial community structure in the ore surface of Zijinshan commercial low-grade copper bioleaching heap was investigated by 16S rRNA gene clone library. For both bacteria and Archaea, 105 clones were sequenced. The dominant bacteria species present in the ore surface were Acidithiobacillus and Leptospirillum, accounting for 51.42% and 48.57%, respectively. However, for the Archaea, only one operational taxonomic unit (OUT) belonged to Ferroplasma acidiphilum. These results indicate that function of genus Acidithiobacillus in the commercial low-grade copper bioleaching heap may be underestimated. More detailed and quantitative information on microbial community structure over time are now under investigation.
Key words:
bioheap; copper; biodiversity; 16S rRNA gene;
1 Introduction
In the last 50 years, heap bioleaching of low-grade copper sulphide minerals has developed into an emerging biotechnology, and is presently at commercial-plant scale. The first commercial plant in China of bio-heap leaching with a capacity of 10 000 t/a started operation at the Zijinshan Copper Mine in the end of 2005[1]. Due to the relatively high proportion of pyrite in the ore (up to 5.8%), the bioleaching plant suffered from excessive acid and iron ions, which decreased the solvent extraction rate and increased the energy consumption.
For process improvement, understanding microbial community within the heap is essential. Within the bacteria assisted heap leaching operated at ambient temperature, bacterial strains related to Acidithiobacillus thiooxidans, Acidithiobacillus ferrooxidans, Acidiphilium cryptum and Leptospirillum ferrooxidans have often been cultured[2-3]. Yet we still have no idea whether cultured strains are the key players in the heap. Up to now, very few culture-independent data are published from commercial copper bioleaching processes. Since previous research found that Leptospirillum ferriphilum was dominant in many bio-heap[4-6], we are very curious about the function of this bacteria group in the Zijinshan heap. Due to the excessive acid and iron ions, bacteria cell number within the leaching solution decreases quickly. Direct cell number in the leaching solution decreases from 106 /mL to less than 105 /mL, resulting in DNA extraction failure. Since there are plenty of bacterial cells associated with ore sludge (106/g), it is valuable to understand microbial community attached to the ore surface which may reflect the community structure of the whole heap.
In this work, by using 16S ribosomal RNA clone library technique, the community structure in the ore sludge was revealed and compared with microbial community in the leaching solution at start-up stage. The functional group in the heap was discussed and correlated to the bioleaching condition.
2 Experimental
2.1 Heap description
Zijinshan Copper Mine is located in the southeast of China. The heap bioleaching plant is now the largest view on the commercial plant. In the first six months of 2008, 1 400 000 t ore with an average copper grade of 0.5% was applied to the heap. The heap consists of three floors with each floor height of 8 m. Up to now, the leaching cycle days are approximately 180 d, and the recovery rate reaches 80%. bioleaching plant in China[1]. Fig.1 shows the overall

Fig.1 Overall view on Zijinshan commercial plant
2.2 Sample preparation, DNA extraction and 16S ribosomal RNA clone library construction
Leaching solution was directly taken from the bottom of the heap for chemical data analysis. The concentration of Cu2+, Fe2+ and Fe3+ were analyzed by the methods described previously[1]. Wet ore sludge samples inside the heap (1 m below the top of the heap) were obtained by using dig machine. Nearly 500 g ore sludge sample was washed by 3 L double distilled water. All the 3 L washed water was centrifuged at 12 000 r/min for 5 min, and the resulting pellet was subjected to microbial analysis. A bead-beating method[7] was used for the extraction of total DNA from culture samples. For the bacteria clone library, amplification of 16S rRNA genes from sludge samples was facilitated by PCR with general primers (27f and 1492r)[8]. For the archaea, archaeal primers Arch21f and 1492r[9] were used. The amplified 16S rRNA genes were inserted into pGEM-T easy vectors (Promega, WI, USA) and transformed into E. coli JM109. The diversity of clone libraries of 16S rRNA genes was examined by endonucleatide restriction digestion, and at least 105 clones from each clone library were sequenced by Sangon Corp. (Shanghai, China). Chimera sequences were detected by the Chimera function at the RDP site (http://rdp.cme.msu.edu/), and all chimera sequences were eliminated.
2.3 Clone library analysis
Sequences were analyzed by using BLAST at the NCBI database (http://ncbi.nlm.nih.gov/BLAST). Alignments of 16S rRNA gene sequences were performed with the CLUSTAL_X program of version 1.64b[10] and corrected manually. A phylip-generated distance matrix was used as the input file to distance-based OTU and richness (DOTUR)[11], which assigns sequences to operational taxonomic units (OTUs) for every possible distance. Rarefaction analysis and the Chao1 non-parametric diversity estimator[12] were applied to the clone library to estimate whether the library had been sequenced enough to extrapolate the total sequence diversity. A neighbour-joining phylogenetic tree was constructed based on evolutionary distances that were calculated with the Kimura two-parameter model. Alignment positions with insertions or deletions were excluded from the calculations. Other diversity indices were calculated by using SPADE (http://chao.stat.nthu.edu.tw).
3 Results
3.1 Classification of clones
For bacteria clone libraries, a total of 105 16S rRNA gene clones are grouped into only 7 OTUs with a distance level of 1%. The 105 OTUs belong to 2 bacterial phyla: Proteobacteria, Nitrospira (Table 1). Of all the identified bacteria sequences, 2 generas are represented which are genus Acidithiobacillus and Leptospirillum presenting 54 and 51 clones, respectively. The collection curve, which plots the number of sequences screened versus the number of OTUs observed with a distance level of 1% and 3% (Fig.2), reaches an asymptote, indicating that the diversity in the library appears to be low enough to ensure sufficient coverage in the screening. This is confirmed by the Chao1 estimator, which computes 9 OTUs (95% confidence intervals, 11 and 14) for the total sequence diversity in the bacteria clone library, and is also supported by the high estimated sample coverage of each clone library (Table 2). While for the archaea community, only one OTU belonging to Ferroplasma acidiphilum is detected, indicating that archaea diversity in the heap is very low.

Fig.2 Collector’s curves
Table 1 Sequencing results of bacteria clone library
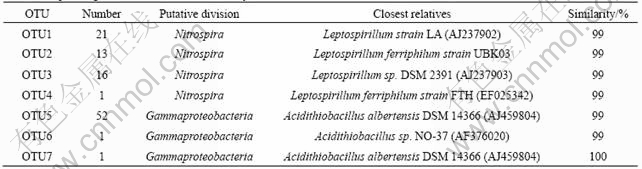
Table 2 Estimated diversity indices for bacterial communities with distance level of 1%

3.2 Relationship between sequences detected and cultured strains
Moleacular analyses of 16S rRNA fragments reveal that all of the sequences retrieved are fell into clusters containing Acidithiobacillus albertensis, Leptospirillum ferriphilum and Ferroplasma acidiphilum strains. Thus, the functional group in the heap should be mainly sulfur oxidizers and iron oxidizers. A comparative phylogenetic analysis of the 16S rRNA genes was performed. The genes are related to a large group of genus Acidithiobacillus and the sequences retrieved are related to genus Acidithiobacillus (Fig.3). Among them, the dominant clone retrieved from the Zijinshan heap is clustered with Acidithiobacillus albertensis and Acidithiobacillus thiooxidans (OTU5 and OTU7), and one sequence (OTU6) belongs to Acidithiobacillus ferrooxidans cluster IV according to Karavaiko’s research[13]. Other bacteria clones are related to Leptospirillum as shown in Fig.4. All the Leptospirillum-isolated sequences from the Zijinshan heap in this research are clustered with Leptospirillum group Ⅱ. This is consistent with previous research [14-15]. Leptospirillum group II is now under the name of Leptospirillum ferriphilum by previous research[15] and is found to be dominant in many bio-heap[4-6], biooxidation tanks[15] and other acid environment[16].
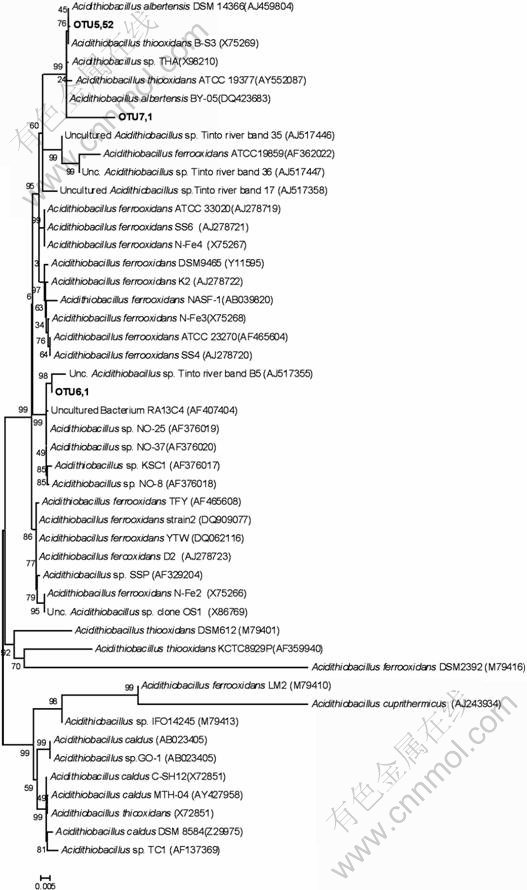
Fig.3 Phylogenetic affiliation of 16S rRNA gene sequences of clone library within genus Acidithiobacillus

Fig.4 Phylogenetic affiliation of 16S rRNA gene sequences of clone library within genus Leptospirillum
3.3 Microbial community associated to ore and in leaching solution
The microbial community of leaching solution from Zijinshan bio-heap was researched at the start-up stage (June 2006). The results show that Leptospirillum is accounted for a high proportion (74% of the clones) (Fig.5), so pyrite leaching in the heap is accelerated by these prevailing iron oxidizers. While in this research, though the heap pH is decreased to 0.78 and high concentration of ferrous and ferric ions exist (Table 3), the dorminance of Acidithiobacillus albertensis at ore surface is revealed (accounting for 51.4% within the ore surface). Acidithiobacillus albertensis is not a well researched microorganism. However, according to the previous information, it may be widely geographical distributed[17]. Due to its behavior in the Zijinshan heap is not quite clear, further research on isolation, distribution and function of this bacteria is now carrying out.
Table 3 Chemical characteristic of heap during two sampling times


Fig.5 Microbial community associated to ore and in leaching solution
4 Discussion
4.1 Microorganism found in Zijinshan bioheap
Many researches have been conducted on the identification of many bioleaching organisms such as Acidithiobacillus ferrooxidans, Leptospirillum ferro- oxidans, Acidithiobacillus thiooxidans[17] and Acidithio- baicillus albertensis[18-19]. In 1980s, Acidithiobaicillus was considered to be the most important micro-organism in commercial bioleaching plants[20]. While in recent findings, by using molecular technology based on PCR amplification and characterization of 16S rRNA gene, Leptospirillum was found to be more important to both uncontrolled (nature) and deliberate mineral bioleaching and biooxidation processes[15, 21]. Attachment to specific minerals would certainly be advantageous to iron-oxidizers growing in flowing water bodies[22].
4.2 Reason of Acidithiobacillus albertensis dominating heap
According to previous research, Leptospirillum and Ferroplasma groups are dominant at the most extreme conditions of lower pH and higher ionic strength[14, 23-24], while Acidithiobacillus and Sulfobacillus are the main components at slightly higher pH and lower conductivity[14]. However, in this research, Acidithiobacillus albertensis is prevailing in the heap atlow pH and higher iron ion concentration, and another two samples from deeper place of the heap revealed the same trend (the data are not shown). Acidithiobacillus albertensis is physiologically similar to Acidithiobacillus thiooxidans[25], representing another branch of sulfur oxidizers. It seems that the predominance of Acidithiobacillus albertensis at the ore surface may be due to the environment pressure. Lower pH and higher ionic strength force these bacteria to excrete even more amount of EPS, which lead them to attach more easily to the ore surface. And when they attach to the ore surface, the micro-environment may be different compared with the plankton cells. Another reason may lie in the redox potential, according to RAWLINGS’ research[21]. When the φh is below 690 mV, Acidithiobacillus ferrooxidans has a faster growth rate than Leptospirillum. Since EPS of Leptospirillum differs significantly from that of cells of the genus Acidithiobacillus[26], it is more easy for Acidithiobacillus albertensis to grow in the mono EPS layer excreted by Acidithiobacillus ferrooxidans. Furthermore, previous research found that the iron- and pyrite-oxidizing activity of a strain of At. ferrooxidans was strongly inhibited when the bacteria were attached to the solid surfaces[27]. This may be due to the formation of sulfur membrane. Acidithiobacillus albertensis has higher sulfur oxidation ability, so it replaces At. ferrooxidans prevailing in the mono EPS layer wrapped outside the ore. Due to the degraded sulfur membrane, the pH is decreased, resulting in the dominance of Leptospirillum.
4.3 Possible ways for control pyrite leaching during copper bioleaching
During the whole research, Sulfobacillus was not detected. According to previous research, Sulfobacillus- like organisms are more competitive with iron oxidizers than with sulfur oxidizers and can maintain leaching system in low redox potential[4, 28]. Moreover, our former research indicated that in the pH range of 1.0- 1.2, Sulfobacillus-like organisms can compete effectively with Leptospirillum, thus reducing pyrite leaching rate. For achieving selective depressing of pyrite leaching during the copper bioleaching, the control of pH and periodical culture and inoculation of Sulfobacillus strains may be choices.
5 Conclusions
1) The dominant bacteria species present in the Zijinshan bio-heap ore surface are Acidithiobacillus and Leptospirillum, accounting for 51.42% and 48.57% respectively.
2) Genus Acidithiobacillus especially Acidithio- bacillus albertensis, may play very important role in the commercial low-grade copper bioleaching heap.
3) Genus Sulfobacillus is not detected in the heap, indicating that for controlling pyrite leaching during the copper bioleaching at Zijinshan heap, inoculation and bio-augmentation of Sulfobacillus might be choices.
References
[1] RUAN R, WEN J, CHEN J. Bacterial heap-leaching: Practice in Zijinshan copper mine [J]. Hydrometallurgy, 2006, 83: 77-82.
[2] GOEBEL B M, STACKEBRANDT E. Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments [J]. Applied and Environmental Microbiology, 1994, 60: 1614-1621.
[3] RAWLINGS D E. The molecular genetics of Thiobacillus ferrooxidans and other mesopholic, acidophilic, chemolithotrophic, iron- or sulfur-oxidizing bacteria [J]. Hydrometallurgy, 2001, 59: 187-201.
[4] DEMERGASSO C, GALLEGUILLOS P, ESCUDERO L V, ZEPEDA V J, CASTILLO D, CASAMAYOR E D. Molecular characterization of microbial populations in a low-grade copper ore bioleaching test heap [J]. Hydrometallurgy, 2005, 80: 241-253.
[5] VASQUEZ M, ESPEJO R T. Chemolithotrophic bacteria in copper ores leached at high sulfuric acid concentration [J]. Applied and Environmental Microbiology, 1997, 63: 332-334.
[6] SAND W, ROHDE K, SOBOTKE B, ZENNECK C. Evaluation of Leptospirillum ferrooxidans for leaching. [J]. Applied and Environmental Microbiology, 1992, 58: 85-92.
[7] OVED T, SHAVIV A, GOLDRATH T, MANDELBAUM R T, MINZ D. Influence of effluent irrigation on community composition and function of ammonia-oxidizing bacteria in soil [J]. Applied and Environmental Microbiology, 2001, 67(8): 3426-3433.
[8] LANE D J. 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics [M]. New York: John Wiley and Sons, 1991: 115-175.
[9] DELONG E F. Archaea in coastal marine environments. [J]. Proc Natl Acad Sci USA, 1992, 89: 5685-5689.
[10] THOMPSON J D, GIBSON T J, PLEWNIAK F. The ClustalX windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools [J]. Nucleic Acids Research, 1997, 24: 4876-4882.
[11] SCHLOSS P D, HANDELSMAN J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness [J]. Applied and Environmental Microbiology, 2005, 71(3): 1501-1506.
[12] CHAO A. Non-parametric estimation of the number of classes in a population [J]. Scand J Stat, 1984, 11: 265-270.
[13] KARAVAIKO G I, KONDRAT’EVA T F, LYSENKO A M, KOLGANOVA T V, AGEEVA S N, MUNTYAN L N, PIVOVAROVA T A. Phylogenetic heterogeneity of the species Acidithiobacillus ferrooxidans [J]. International Journal of Systematic and Evolutionary Microbiology, 2003, 53: 113-119.
[14] BOND P L, DRUSCHEL G K, BANFIELD J F. Comparison of acid mine drainage microbial communities in physically and geochemically distinct ecosystems [J]. Applied and Environmental Microbiology, 2000, 66: 4962-4971.
[15] CORAM N J, RAWLINGS D E. Molecular relationship between two groups of the genus Leptospirillum and the finding that Leptospirillum ferriphilum sp. nov., dominates in South African commercial biooxidation tanks that operate at 40 ℃ [J]. Applied and Environmental Microbiology, 2002, 68: 838-845.
[16] GOEBEL B, STACKEBRANDT E. Cultural and phylogenetic analysis of mixed microbial populations found in naturaland commercial bioleaching environments [J]. Applied and Environmental Microbiology, 1994, 60: 1614-1621.
[17] KELLY D P, HARRISON A P. Genus Thiobacillus Beijerinck [M]// Bergey’s Manual of Systematic Bacteriology. Baltimore: Williams & Wilkins, 1989.
[18] BRYANT R D, MCGROARTY K M, COSTERTON J W, LAISHLEY E J. Isolation and characterization of a new acidophilic Thiobacillus species (T.alberfis). [J]. Can J Microbiol, 1983, 29: 1159-1170.
[19] XIA Jin-lan, HE Huan, YANG Yu, LIU Xue-duan, QIU Guan-zhou. A new strain Acidithiobacillus albertensis BY-05 for bioleaching of metal sulfides ores [J]. Trans Nonferrous Met Soc China, 2007, 17: 168-175.
[20] LUNDGREN D G, SILVER M. Ore leaching by bacteria [J]. Annu Rev Microbiol, 1980, 34: 263-283.
[21] RAWLINGS D E, TRIBUTSCH H, HANSFORD G S. Reasons why ‘Leptospirillum’-like species rather than Thiobacillus ferrooxidans are the dominant iron-oxidizing bacteria in many commercial processes for biooxidation of pyrite and related ores [J]. Microbiology, 1999, 145: 5-13.
[22] G HAURI M A, OKIBE N, JOHNSON D B. Attachment of acidophilic bacteria to solid surfaces: The significance of species and strain variations [J]. Hydrometallurgy, 2007, 85: 72-80.
[23] EDWARDS K J, GIHRING T M, BANFIELD J F. Seasonal variations in microbial populations and environmental conditions in anextreme acid mine drainage environment [J]. Applied and Environmental Microbiology, 1999, 65: 3627-3632.
[24] OKIBE N, GERICKE M, HALLBERG K B, JOHNSON D B. Enumeration and characterization of acidophilic microorganisms isolated from a pilot plant stirred-tank bioleaching operation [J]. Applied and Environmental Microbiology, 2003, 69: 1936-1943.
[25] SCHIPPERS A. Microorganism involved in bioleaching and nucleic acid-based molecular methods for their identification and quantification [M]// Microbial Processing of Metal Sulfides. Dordrecht: Springer, 2007.
[26] HARNEIT K, GOKSEL A, KOCK D, KLOCK J H, GEHRKE T, SAND W. Adhesion to metal sulfide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans [J]. Hydrometallurgy, 2006, 83: 245-254.
[27] WAKAO N, MASHINA M, SAKURAI Y, SHIOTA H. Bacterial pyrite oxidation (III): Adsorption of Thiobacillus ferrooxidans cells to solid surfaces and its effect on iron release from pyrite [J]. J Gen Appl Microbiol, 1984, 30: 63-77.
[28] ADIBAH YAHYA D B J. Bioleaching of pyrite at low pH and low redox potentials by novel mesophilic Gram-positive bacteria [J]. Hydrometallurgy, 2002, 63: 181-188.
Foundation item: Project(2007AA060901) supported by the High-tech Research and Development Program of China; Project(2004CB619205) supported by the National Basic Research Program of China
Corresponding author: WEN Jian-kang; Tel: +86-10-82241313, Fax: +86-10-62014824; E-mail: kang3412@126.com
(Edited by YUAN Sai-qian)


