
Solution-deposited Li4Ti5O12 thin films as anode for lithium ion battery
WU Xian-ming(吴显明), XIAO Zhuo-bing(肖卓炳), MA Ming-you(麻明友), CHEN Shang(陈 上),
HE Ze-qiang(何则强), LIU Jian-ben(刘建本)
College of Chemistry and Chemical Engineering, Jishou University, Jishou 416000, China
Received 20 April 2006; accepted 30 June 2006
Abstract: The technique of solution deposition was employed to prepare Li4Ti5O12 thin film using lithium acetate and TiO(C4H9)4 as starting materials. The structural and electrochemical properties of the thin films were studied by X-ray diffraction, cyclic voltammetry, galvanostatic charge-discharge experiments, and potential step technique. The results show that the thin film prepared by this method is of pure phase with a spinel framework structure. The capacity of the thin film annealed at 750 ℃ for 1 h is approximately 57 μA?h/(cm2?mm). The film possesses excellent cycling behavior with a 0.08% capacity loss per cycle after being cycled 50 times. Potential step technique shows that the average chemical diffusion coefficient of lithium ion in the thin film is approximately 4.5×10-11 cm2/s.
Key words:
heat treatment; deposition; thin films; coatings;
1 Introduction
Li4Ti5O12, possessing good reversibility and the characteristics of no structural change (zero-strain insertion material) in the charge-discharge process[1], was always demonstrated as a good candidate as negative electrode for solid-state lithium-ion batteries. Li4Ti5O12 reversibly inserts 0.7-1.0 Li at 1.55 V. Therefore, this material can be coupled with 4.0 V electrodes as LiMn2O4 and LiCoO2 to provide a cell with an operating voltage of approximately 2.5 V which is twice that of nickel-cadmium or nickel-metal hydride cell.
Recently, thin-film lithium-ion batteries have drawn a lot of attention[2-5] due to their many possible applications, such as smart cards, CMOS-based integrated circuits and microdevices[6-8]. A lot of thin films that can be used as electrode material for thin-film lithium-ion batteries, such as LiMn2O4[9-11], LiCoO2[12, 13], SnO2[14, 15], etc have been studied. But there were few reports about Li4Ti5O12 thin film. In this study, Li4Ti5O12 thin film was prepared by the solution deposition and its properties were investigated.
2 Experimental
Stoichiometric amount of lithium acetate was dissolved in 2-methoxyethanol. Then TiO(C4H9)4 was added into the solution drop by drop under constant stirring. Dust and other suspended impurities were removed from the solution by filtering through 0.2 μm syringe filters, and thus obtained Li4Ti5O12 precursor solution. The wet film was formed by spin coating Li4Ti5O12 precursor solution onto substrates (Si substrates for structural analysis and Pt-coated substrates for electrochemical measurements). Then the wet films were heated at 380 ℃ in air for 15 min at a heating rate of 10 ℃/min to remove the solvent and other organic substances. The deposition and heat treatment procedure were repeated to prepare films with the desired thickness. The multilayered films were finally annealed at high temperatures in air to make them crystallize.
The thermal decomposition behavior of the Li4Ti5O12 precursor powder was examined by the thermogravimetric analysis at a heating rate of 10
℃/min. The precursor powder was derived from heating the Li4Ti5O12 precursor solution at 140 ℃ for 1 h. Phase identification was carried out with an X-ray diffractometer (XRD).
For electrochemical measurements, 0.3 mm-thick Li4Ti5O12 thin films annealed at 750 ℃ for 1 h were placed in an open beaker cell containing electrolyte of 1 mol/L LiPF6/EC- DMC solution. Lithium metal was used as both auxiliary and reference electrodes. The entire cell was assembled in an argon-filled glove box. Galvanostatic charge-discharge experiments were conducted between 1.2 V and 2.2 V under a constant current density of 100 μA/cm2. The cyclic voltammetry was performed at the scan rate of 1 mV/s, and potential step technique is employed to determine chemical diffusion coefficient of lithium ion in Li4Ti5O12 thin film.
3 Results and discussion
Fig.1 shows the thermogravimetric curve of Li4Ti5O12 precursor gel. The mass loss is about 18% when the heating temperature reaches 110 ℃, which is caused by the volatilization of the absorbed water and organic substance. The main mass loss starts at 200 ℃, which is caused by the combustion of lithium acetate and other substances. The mass loss terminated at about 500 ℃, indicating that the relevant substances are completely decomposed.
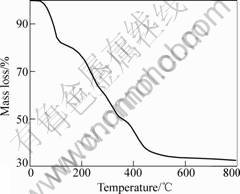
Fig.1 Thermogravimetric curve of Li4Ti5O12 precursor gel
The analysis of crystal structure of the prepared thin film by XRD is shown in Fig.2. As shown in Fig.2, for the thin film annealed at 380 ℃ and 550 ℃, except the substrate peaks, no other peaks can be observed, indicating that these films are amorphous. However, when the annealing temperature reaches 650 ℃, four other peaks appear. These four diffraction lines indexed in terms of (h, k, l) ((111), (311), (400), and (331) reflections) are assigned for a face-centered cubic lattice, space group of Fd3M, suggesting that the films have a spinel framwork structure of Li4Ti5O12. In the structure, some lithium ions are located at the tetrahedral 8(a) sites, other lithium and titanium ions are distributed at octahedral 16(d) sites with the mole ratio of Li/Ti=1/5, while oxygens are located at the 32(e) sites. Therefore, Li4Ti5O12 can be described as ![]() .
.
The infrared spectra of Li4Ti5O12 derived from different conditions are shown in Fig.3. For the sample heated at 380 ℃ for 15 min, two peaks at the wavenumber around 1 500 cm-1 are observed. The two peaks are the stretching vibration of C—O, which belong
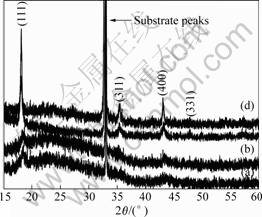
Fig.2 X-ray diffraction patterns of prepared thin films annealed under different conditions: (a) 380℃,15 min; (b) 550 ℃, 1 h; (c) 650 ℃, 1 h; (d) 750 ℃, 1 h
to ![]() . This suggests that there is the combustion of lithium acetate, which leads to the formation of Li2CO3. The vibrations become weaker as annealing temperature increases and disappear when the heating temperature reaches 750 ℃. The stretching vibrations (at about 460 cm-1 and 650 cm-1) of Ti—O band become stronger with the increase of heating temperature. This implies that the strength of Ti—O increases with the elevation of heating temperature.
. This suggests that there is the combustion of lithium acetate, which leads to the formation of Li2CO3. The vibrations become weaker as annealing temperature increases and disappear when the heating temperature reaches 750 ℃. The stretching vibrations (at about 460 cm-1 and 650 cm-1) of Ti—O band become stronger with the increase of heating temperature. This implies that the strength of Ti—O increases with the elevation of heating temperature.
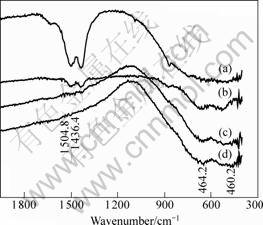
Fig.3 Infrared spectra of Li4Ti5O12 derived under different conditions: (a) 380 ℃, 15 min; (b) 550 ℃, 1 h; (c) 650 ℃, 1 h; (d) 750 ℃, 1 h
The cyclic voltammogram of a Li4Ti5O12 thin film is shown in Fig.4. As seen in Fig.4, one reduction and oxidation process appear, characterized by the corresponding cathodic and anodic peak potentials respectively, which locate at 1.54 V and 1.60 V. The difference between the cathodic and anodic peak potential is about 59 mV, indicating that the reversibility of the thin film is excellent.
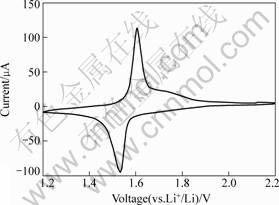
Fig.4 Cyclic voltammogram of Li4Ti5O12 thin film
The charge and discharge curves of Li4Ti5O12 thin film are shown in Fig.5. As shown in Fig.5, the charge and discharge curves are very flat and their working potentials are around 1.55 V. The thin film delivers the discharge capacity of 57 μA?h/(cm2?μm), and shows a high coulombic efficiency of 98%.
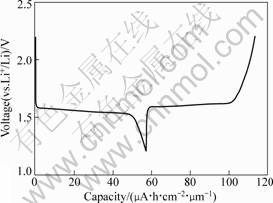
Fig.5 Charge and discharge curves of Li4Ti5O12 thin film
Fig.6 shows the capacity of Li4Ti5O12 thin film discharging at different current densities. As the discharge current density increases from 50 μA/cm2 to 400 μA/cm2, the capacity deduction of the film is not significant. This indicates that the influence of the discharge current density on the capacity of Li4Ti5O12 thin film is small, which is caused by two reasons. The one is that the thin-film electrode is very thin, and therefore the electrode possesses small resistance. The second is that the electrochemical testing system employs liquid electrolyte, which possesses relatively higher ionic conductivity, and thus results in lower internal resistance.
The variation of the capacity as a function of cycle number for the Li4Ti5O12 thin film is shown in Fig.7. As shown in Fig.7, the capacity fading is very small. The capacity loss per cycle after being cycled for 50 times is only about 0.08%, suggesting that the film has good rechargeability. The excellent cycling behavior of the film is associated with the reaction of Li4+xTi5O12 in the charge and discharge process. During the cycling, lithium intercalation and deintercalation take place according to the following reaction:
Li4Ti5O12+3Li++3e-=Li7Ti5O12
The rock-salt-type Li7Ti5O12 has almost the same lattice parameters as those of Li4+xTi5O12, which results in good capacity maintenance during cycling.
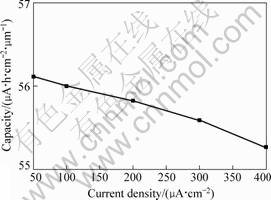
Fig.6 Capacity of Li4Ti5O12 thin film as a function of discharge current density
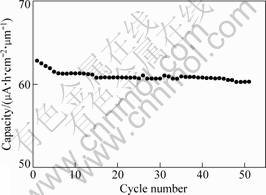
Fig.7 Variation of capacity as a function of cycle number for Li4Ti5O12 thin film
Potential step is one of techniques to determine chemical diffusion coefficient. In this method, the current (I) generated due to an applied voltage step, is measured as a function of time (t). The measured current decays as the lithium ion diffuses through the electrode. The step is ended when the current becomes less than a specified minimum, for example, 1% of the maximum current at the onset of the applied potential. In the measurement, the applied potential step is 0.05 V (vs. Li/Li+)(1.45?1.50 V, 1.50?1.55 V, 1.55?1.60 V). Fig.8 shows the I-t and I-t -1/2 curves. By assuming that the semi-finite diffusion of lithium ion in the electrode is the rate-determining procedure, the diffusion coefficient DLi of lithium ion in the thin-film electrode can be determined by the following Cottrell equation[16]:
![]()
where n is the number of redox reaction, F is the Faraday constant, c0 is the lithium ion concentration in the solid thin film electrode, which can be calculated from the open circuit voltage and assuming that the density of the thin film equals its theoretical density. From Cottrell equation and Fig.8(b), the diffusion coefficient of lithium ion in the electrode can be calculated. The average diffusion coefficient of lithium ion in Li4Ti5O12 thin films tested by this method is approximately 4.5×10-11 cm2/s.
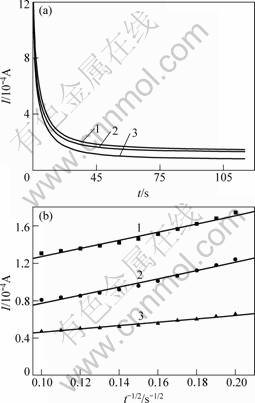
Fig.8 Variation of current as a function of t (a) and t-1/2 (b): 1—1.45-1.50 V; 2—1.50-1.55 V; 3—1.55-1.60 V
4 Conclusions
Li4Ti5O12 thin film was prepared by solution deposition technique. The thin film prepared by this method is of pure phase. The thin film annealed at 750 ℃ for 1 h delivers a capacity of approximately 57 μA?h/(cm2?μm) and possesses excellent cycling behavior with a 0.08% capacity loss per cycle after being cycled for 50 times. The average chemical diffusion coefficient of lithium ion in the thin film is approximately 4.5×10-11 cm2/s.
References
[1] NUSPL G, YOSHIZAWA K, YAMABE T. Lithium intercalation in TiO2 modifications[J]. J Mater Chem, 1997, 7: 2529-2536.
[2] WEST W C, WHITACRE J F, LIM J R. Chemical stability enhancement of lithium conducting solid electrolyte plates using sputtered LiPON thin films[J]. J Power Sources, 2004, 126: 134-138.
[3] LEE S J, BAE J H, LEE H W, BAIK H K, LEE S M. Electrical conductivity in Li-Si-P-O-N oxynitride thin-films[J]. J Power Sources, 2003, 123: 61-64.
[4] KIM M K, CHUNG H T, PARK Y J, KIM J G, SON J T, PARK K S, KIM H G. Fabrication of LiCoO2 thin films by sol–gel method and characterisation as positive electrodes for Li/LiCoO2 cells[J].J Power Sources, 2001, 99: 34-40.
[5] BATES J B, DUDNEY N J, NEUDECKER B J, HART F X, JUN H P, HACKNEY S A. Preferred orientation of polycrystalline LiCoO2 films[J]. J Electrochem Soc, 2000, 147: 59-70.
[6] JONES S D,AKRIDGE J R. Development and performance of a rechargeable thin-film solid-state microbattery[J]. J Power Sources, 1995, 54: 63-67.
[7] BATES J B, DUDNEY N J, NEUDECKER B, UEDA A, EVANS C D. Thin-film lithium and lithium-ion batteries[J]. Solid State Ionics, 2000, 135: 33-45.
[8] PARK Y J, KIM J G, KIM M K, CHUNG H T, UM W S, KIM M H, KIM H G. Fabrication of LiMn2O4 thin films by sol–gel method for cathode materials of microbattery[J]. J Power Sources, 1998, 76: 41-47.
[9] MATSUDA K, TANIGUCHI I. Relationship between the electrochemical and particle properties of LiMn2O4 prepared by ultrasonic spray pyrolysis[J]. J Power Sources, 2004, 132: 156-160.
[10] SON J T, KIM H G, PARK Y J. New preparation method and electrochemical property of LiMn2O4 electrode[J]. Electrochimica Acta, 2004, 50: 453-459.
[11] ZHANG Y L, SHIN H C, DONG J, LIU M. Nanostructured LiMn2O4 prepared by a glycine-nitrate process for lithium-ion batteries[J]. Solid State Ionics, 2004, 171: 25-31.
[12] NAGASUBRAMANIAN G, DOUGHTY D H. Electrical characterization of all-solid-state thin film batteries[J]. J Power Sources, 2004, 136: 395-400.
[13] DUDNEY N J, JANG Y I. Analysis of thin-film lithium batteries with cathodes of 50 nm to 4 μm thick LiCoO2[J]. J Power Sources, 2003, 119-121: 300-304.
[14] SANTOS-PE?A J, BROUSSE T, S?NCHEZ L, MORALES J, SCHLEICH D M. Antimony doping effect on the electrochemical behavior of SnO2 thin film electrodes[J]. J Power Sources, 2001, 97-98: 232-234.
[15] CHOI S H, KIM J S, YOON Y S. Fabrication and characterization of SnO2-RuO2 composite anode thin film for lithium ion batteries[J]. Electrochimica Acta, 2004, 50: 547-552.
[16] FU Z W, QIN Q Z. Electrochemical and electrochromic properties of niobium oxide thin films fabricated by pulsed laser deposition[J]. J Electrochem Soc, 1999, 146: 3914-3918.
Corresponding author: WU Xian-ming; Tel: +86-743-8563911; E-mail: xianmingwu@163.com


