
![]()

Trans. Nonferrous Met. Soc. China 22(2012) 711-716
Electrochemical behavior of Pb(II) in LiCl-KCl-MgCl2-PbCl2 melts on Mo electrode
ZHANG Mi-lin, CHEN Li-jun, HAN Wei, YAN Yong-de, CAO Peng
Key Laboratory of Superlight Materials and Surface Technology (Ministry of Education),
College of Material Science and Chemical Engineering, Harbin Engineering University, Harbin 150001, China
Received 14 April 2011; accepted 23 June 2011
Abstract:
Cyclic voltammetry and chronopotentiometry were used to study the reaction mechanism of Pb(II) and the co-deposition of Pb, Mg and Li on molybdenum electrodes in LiCl-KCl-PbCl2-MgCl2 melts. The diffusion coefficient of lead ions in the melts was determined by different electrochemical techniques. The results obtained by cyclic voltammetry and chronopotentiometry indicated that the underpotential deposition of lithium on pre-deposited Pb leads to the formation of a liquid Li-Pb alloy, and the Mg-Li-Pb alloys are formed after the addition of MgCl2. X-ray diffraction confirmed that in the Mg-Li-Pb alloy, PbLi3, Mg2Pb and Li7Pb2 phases exist by galvanostatic electrolysis at 6.21 A/cm2 for 2 h at 873 K and the phases can be controlled by changing the concentration of PbCl2 and MgCl2.
Key words:
LiCl-KCl system; cyclic voltammetry; chronopotentiometry; diffusion coefficient; molten salts electrolysis;
1 Introduction
Pb and its alloys are used in most of automotive, nuclear shielding material and industrial batteries. Indeed, lead alloys are usually used instead of pure lead for alloying with insignificant amounts of some metals can greatly improve the corrosion characteristics of the material [1].
Researches on the electrochemical and aging behaviors of Mg-Pb, Ag-Pb, Ca-Pb, Sn-Pb, Al-Pb, etc, alloys have been studied. BUI et al [2] studied the electrochemical properties and corrosion resistance of Pb-Ag and Pb-Ca, and Pb-Ag-Ca alloys with different content of the alloying components, and compared them with those of the anodes used in the electro-extraction of Zn, binary cast, alloy of a Pb-Ag 1% composition. ABRAMOV et al [3] produced an alloy with uniform distribution of the Pb-phase over the height of the ingot to study its mechanical and antifriction properties. The Mg in Mg-Pb alloys used as cathode was analyzed by increasing the current density from 0.48 to 1.98 A/cm2 during the electrolysis [4,5]. In the Mg-Pb system the solid solutions both in the Mg and Pb terminal exist, but there is little research about it [5]. Adding different quantity of Pb to AZ61 or AZ91 had been studied. The result showed that the addition of a small Pb can clearly suppress the precipitation of Mg17Al12 and the diffusion of Mg and Al atoms, but the addition of excessive Pb will decrease the corrosion resistance and flame-retarded property of Mg-Al alloy[6]. MUKHOPADHYAY and SHARON [7] discussed the effects of Sn-doping in Pb-Sn alloy oxide, and various parameters on the semiconductor-electrolyte junction behavior by Butler-Gartner model.
At present, the primary methods to prepare metal and its alloys include metal casting, thermic reduction method and molten salt electrolysis method [8]. However, the metal casting and thermic reduction process have many disadvantages, such as complicated production process, high-energy consumption, serious metal burning and inhomogeneous alloy composition. On the contrary, molten salt electrolysis process is an effective method for the preparation of metal and its alloys. Firstly, the process can be carried out with the simple apparatus. Secondly, the high-melting point metals can be produced at a low temperature. Thirdly, the composition of alloys can be controlled easily [9]. So, it is necessary to study the electrochemical behavior of metal ions in the melts. Our group have published a series of papers on this topic. For example, Mg-Li alloys with different contents via a two-electrode cell in the eutectic LiCl-KCl were electrolyzed at 693-783 K [10], the electrochemical behaviors of Mg-Li, Zr, Zn, and Al alloys on Mg or Mo electrode in the LiCl-KCl melts were investigated and produced [11-14].
In the present work, the electrochemical behavior of Pb(II) on molybdenum electrode was studied in the LiCl-KCl-PbCl2-MgCl2 melts at 873 K with a series of electrochemical techniques, such as cyclic voltammetry and chronopotentiometry. The Mg-Li-Pb alloys were prepared by galvanostatic electrolysis at 6.21 A/cm2 and 873 K for 2 h, and analyzed with X-ray diffraction (XRD).
2 Experimental
2.1 Materials
The LiCl and KCl used in the experiments were dehydrated at 573 K and 873 K for about 24 h before electrolysis to remove residual water. Pre-electrolysis was conducted at -2.00 V (vs Ag/Ag+) for 4 h to remove the metal ion impurities in the melts. Solutions of Pb(II) and Mg(II) were prepared by direct additions of solid PbCl2 (analytical grade) and MgCl2 (analytical grade). The electrolysis was carried out under the protection of a carefully purified and dehydrated argon atmosphere.
A graphite rod (d6 mm×10 mm) with high purity and density was used as a counter electrode. The working electrode was molybdenum wire (d1 mm×10 mm). The lower end of the molybdenum working electrode was polished thoroughly with SiC paper and electropolished in HCl aqueous solution, and then cleaned in ethanol using ultrasound. The reference electrode was Ag wire (diameter 1 mm), which was also electropolished in the HCl aqueous solution, and then cleaned in ethanol using ultrasound, immersed in the LiCl-KCl eutectic melts containing AgCl (0.075 mol/kg) in a Pyrex tube.
2.2 Preparation of Mg-Li-Pb alloys
The Mg-Li-Pb alloys were prepared by galvanostatic electrolysis containing different contents of PbCl2 and MgCl2 at 6.21 A/cm2 and 873 K for 2 h. Mg-Li-Pb alloy was a small liquid drop with spherical shape clung on the molybdenum electrode at the beginning. When the nucleation of Mg-Li-Pb alloy on molybdenum electrode grew to some extent, the liquid Mg-Li-Pb alloy departed from the molybdenum electrode due to the effect of surface tension. Majority of Mg-Li-Pb alloys existed in the form of liquid alloys with spherical shape in melts. After electrolysis, the Mg-Li-Pb alloys were extracted from the melts. All of the samples were washed in hexane (99.8% purity) in an ultrasonic bath to remove salts, and then stored in a glove box for analysis.
2.3 Electrochemical apparatus and auxiliary techniques
All of the electrochemical studies, such as cyclic voltammetry pulse techniques and chronopotentiometry, were performed with IM6e electrochemical workstation and the measurement was controlled with the IM6e software package (Zahner Co., Ltd). The Mg-Li-Pb alloy samples obtained by the electrolysis were analyzed by XRD (Multi Flex TTR-III; Rigaku Industrial Corp. Ltd.) using Cu Kα radiation at 40 kV and 150 mA and inductively coupled plasma-atomic emission spectrometry (ICP-AES).
3 Results and discussion
3.1 Cyclic voltammetry
Figure 1 shows typical cyclic voltammograms of the LiCl-KCl-PbCl2-MgCl2 melts on a molybdenum electrode. Curve 1 represents the voltammogram before the addition of PbCl2 and MgCl2, only one couple of cathodic and anodic signals (φ/φ′) which correspond to the deposition and dissolution of liquid Li are observed. Curves 2 and 3 show the voltammograms measured in the LiCl-KCl-PbCl2(1%) and LiCl-KCl-PbCl2(1%)- MgCl2(3%) melts, respectively. Three couples of cathodic and anodic signals are observed except the deposition and dissolution signals of liquid Li (φ/φ′). The deposition potential of Pb is more positive than that of Mg in a chloride system [15]. Peaks of C-C′ and B-B′ in the forward scan are ascribed to the deposition and dissolution of Pb and Mg, respectively. In curve 1, the reduction of Li(I) starts at about -2.34 V; in curve 2, the reductions of Pb(II) and Li(I) start at -0.35 V and -2.31 V. The deposition potential of Li before and after the addition of PbCl2 has nearly no change. Peak couples of a-a′ and b-b′ in curve 2 are thought to be the deposition and dissolution signals of Li-Pb compounds, and their deposition potentials are -2.19 V and -2.08 V, respectively. After the addition of MgCl2, the reductions of Pb(II), Mg(II) and Li(I) start at -0.21 V, -1.50 V and -2.19 V. The deposition potentials of Li and Pb are more positive than those obtained before the addition of MgCl2. The potential shift is due to a lowering activity of the deposited metal in other foreign substrate. The foreign substrate is probably Mg-Pb alloys pre-deposited on the molybdenum electrode. Peaks of a-a′ and b-b′ disappear in curve 3, which are probably hid by the huge current signals of cathode and anode, A-A′.
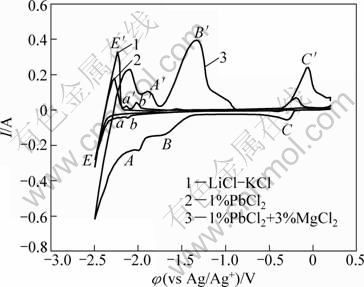
Fig. 1 Cyclic voltammograms on Mo electrode before and after addition of different concentrations of PbCl2 and MgCl2 (scan rate: 0.1 V/s)
Cyclic voltammograms at various scan rates were obtained on a molybdenum electrode in the LiCl- KCl-PbCl2 (1%, 6.92×10-5 mol/cm3) at 873 K in the potential range from 0 to -0.5 V (vs Ag/Ag+) (Fig. 2). There are only one couple of current peaks in the voltammogram and no significant difference in shape among the voltammograms at various scan rates. However, for a reversible electrode reaction involving the deposition of an insoluble substance, |φp-φp/2| (φp is the peak potential; φp/2 is the half-peak potential) should have a value of 0.7725RT/(nF) or 0.021 V [16]. But in this case, the value of |φp-φp/2| was found to exceed 0.021 V. Besides, the cathodic peak shifted a little towards the negative potential with the increase of scan rates, which indicates that the reduction process of Pb(II) may be not completely reversible [17]. Based on the result of the cyclic voltammograms obtained on the molybdenum electrode, it seems that the electroreduction of Pb(II) proceeds via one step process at 873 K.
Figure 3 gives the plot of Ip against ν1/2. It can be
seen that Ip and ν1/2 show a linear relationship, which indicates that the electrode process of Pb(II) is controlled by the mass transfer. The diffusion coefficient can be calculated by the Berzins-Delahay equation for soluble- insoluble systems[15]:
![]() (1)
(1)
where n is the number of exchanged electrons; F is Faraday’s constant (96.485 kC/mol); S is the electrode surface area in cm2; c0 is the solute concentration in mol/cm3; D is the diffusion coefficient in cm2/s1; ν is the potential sweep rate in V/s; R is the mole gas constant; and T is the thermodynamic temperature in K.
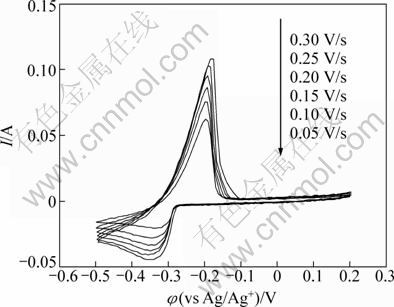
Fig. 2 Cyclic voltammograms on Mo electrode in LiCl-KCl- PbCl2 melts with various scan rates (SMo=0.3219 cm2)
The calculation of the diffusion coefficient using Eq. (1) yields DPb(II)=2.81×10-5 cm2/s.
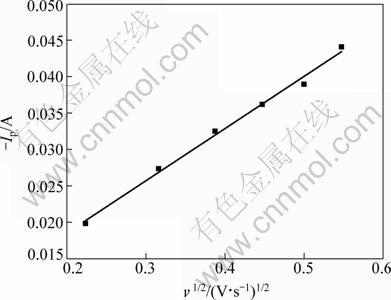
Fig. 3 Variation of cathodic peak current with scan rate (SMo=0.3219 cm2)
3.2 Chronopotentiometry
Chronopotentiometric experiments were carried out to further study the electrochemical behavior of Pb(II). Figure 4 presents the chronopotentiograms on a Mo electrode in LiCl-KCl-PbCl2(1%, 6.92×10-5 mol/cm3) at 873 K. The first plateau (1) is associated with the reduction of Pb. When the cathodic current is -140 mA, four potential plateaus are seen in the potential from 0 V to -2.5 V. Plateaus (2) and (3) are associated with the Li-Pb alloys. The plateau at -2.37 V is due to the reduction of lithium ions. It is obvious that the potential ranges for deposition of Li, and Pb are the same as those observed in the cyclic voltammograms in Section 3.1.
Figure 5 gives the plot of I against τ-1/2. It can be seen that I and τ-1/2 show a linear relationship. The diffusion coefficient of Pb(II) in the eutectic LiCl-KCl can be calculated with the Sand’s equation [17]:
![]() (2)
(2)
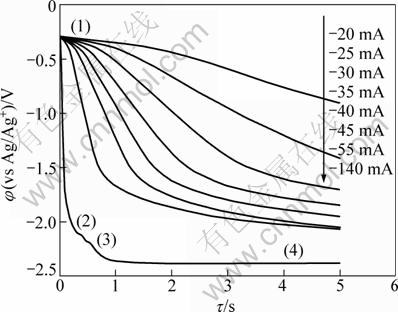
Fig. 4 Chronopotentiograms on Mo electrode in LiCl-KCl- PbCl2 with various currents (SMo=0.3219 cm2)
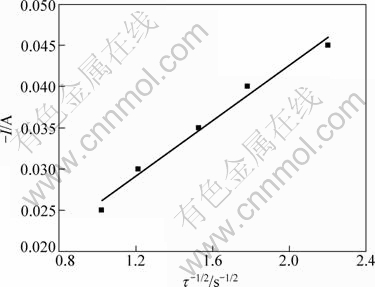
Fig. 5 Plot of I vs τ-1/2 in LiCl-KCl-PbCl2 melts (SMo=0.3219 cm2)
where τ is the transition time measured in the chrono- potentiograms determined by the duration of reduction of Pb(II) between -25 mA and -45 mA using the method described in Ref. [18]. The calculation of the diffusion coefficient of Pb(II) using Eq. (2) yields DPb(II)= 1.95×10-5 cm2/s, which is in the same scalar with the value obtained by cyclic voltammetry.
Figure 6 shows chronopotentiograms obtained at different currents for LiCl-KCl-MgCl2 (3%)-PbCl2(1%) melts (c(PbCl2)=6.70×10-5 mol/cm3, c(MgCl2)= 5.88×10-4 mol/cm3) at 873 K. Between -25 mA and -65 mA of cathodic current, the curves exhibit two potential plateaus, which are associated with the reduction of Pb and Mg. Three potential plateaus are seen in the potential from 0 to -2.5 V in the chronopotentiogram when the cathodic current was -250 mA. The potentials of -1.56 V (1), -1.98 V (2) and -2.16 V (3) are corresponded to the cathodic peaks of B and A, and the Li(I) electroreduction potential of the cyclic voltammograms (Fig. 1), respectively. The potential plateau of the electroreduction of Pb(II)/Pb disappeared at the larger current density (0.932 A/cm2).
It is obvious that the potential ranges for deposition of Pb, Mg and Li correspond to those observed in the cyclic voltammograms (Fig. 1).
Figure 7 gives the plot of I against τ-1/2. It can be seen that I and τ-1/2 show a linear relationship. The diffusion coefficient of Pb(II) in the LiCl-KCl-MgCl2, DPb(II)=2.26×10-5 cm2/s can be calculated with Eq. (2), which is in good agreement with the above results. Mg(II) has no obvious influence on the diffusion coefficient of Pb(II) in this system.
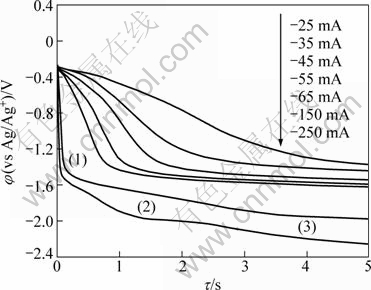
Fig. 6 Chronopotentiograms in LiCl-KCl-MgCl2-PbCl2 melt (SMo=0.3219 cm2)
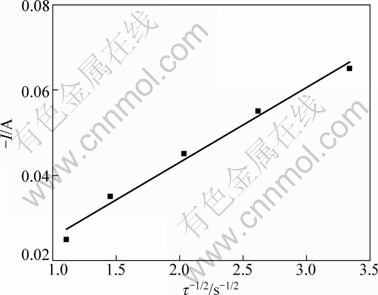
Fig. 7 Plot of I vs τ-1/2 for reduction of Pb(II) in LiCl- KCl-MgCl2 (SMo=0.3219 cm2)
3.3 Galvanostatic electrolysis and characterization of deposits
In order to confirm the formation of Mg-Li-Pb alloys, galvanostatic electrolysis was conducted in the LiCl-KCl containing MgCl2 and PbCl2 with different concentrations on molybdenum electrodes at 6.21 A/cm2 for 2 h at 873 K. The conditions are based on the same method to produce Mg-Li alloys [11].
Figure 8 shows the XRD patterns of Mg-Li-Pb alloys obtained by galvanostatic electrolysis from the LiCl-KCl melts containing (6%-7%) MgCl2 and (0.2%-0.5%) PbCl2. All Mg-Li-Pb alloys contain β-Li phase. However, with the increase of PbCl2 concentration (from 0.2% to 0.5%) in LiCl-KCl-MgCl2 (6%) melts at a constant current, a new Pb phase appears in Fig. 8(b). Phase structure of the Mg-Li-Pb alloys obviously become complicated (PbLi3, Mg2Pb and Li7Pb2 phases appear) from the result of XRD, as the concentration of MgCl2 was increased. From these results, the phases variation of the Mg-Li-Pb alloys depends on the change of MgCl2 and PbCl2 concentrations.
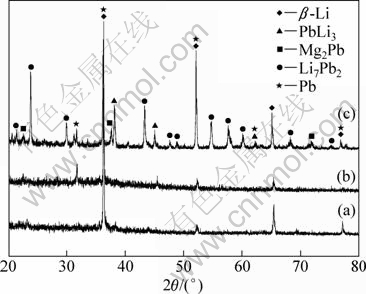
Fig. 8 XRD patterns of deposits obtained by galvanostatic electrolysis on Mo electrodes at 873 K in LiCl–KCl melts containing: (a) 6% MgCl2 and 0.2% PbCl2; (b) 6% MgCl2 and 0.5% PbCl2; (c) 7% MgCl2 and 0.5% PbCl2
In order to obtain the contents of Mg, Li and Pb in the alloys, we carried out the ICP analysis of the alloys. The ICP analyses of all samples obtained by galvanostatic electrolysis are listed in Table 1. The ICP results show that the chemical compositions of Mg-Li-Pb alloys are consistent with the phase structures of the XRD patterns. By galvanostatic electrolysis, the lead content of Mg-Li-Pb alloys increases with increasing PbCl2 concentration in LiCl-KCl-MgCl2 melts. The higher the MgCl2 concentration in the LiCl-KCl melts with equivalent PbCl2 concentration at a constant current, the lower the lithium content of Mg-Li-Pb alloys. Based on these results, it can be concluded that the lithium and lead contents of Mg-Li-Pb alloys can be adjusted by changing the MgCl2 and PbCl2 concentrations.
Table 1 ICP analyses of samples obtained by galvanostatic electrolysis (6.21 A/cm2) on Mo electrode (S=0.3219 cm2) in LiCl-KCl melts containing different MgCl2 and PbCl2 concentrations at 873 K for 2 h
4 Conclusions
1) The electrochemical behavior of Pb(II) was studied by cyclic voltammetry and chronopotentiometry. Cyclic voltammograms showed that the reductions of Pb(II) were detected at -0.35 V and -0.21 V in LiCl-KCl melt before and after the addition of MgCl2, respectively.
2) The diffusion coefficients of Pb(II) ions in the LiCl-KCl before and after the addition of MgCl2 were determined by cyclic voltammetry and chrono- potentiometry to be approximately.
3) Mg-Li-Pb alloys have been successfully prepared by co-deposition at current density 6.21 A/cm2 at temperature 873 K for 2 h. XRD patterns indicated that the main phases of the alloys are β-Li. Pb, PbLi3, Mg2Pb and Li7Pb2 appeared when the concentrations of MgCl2 and PbCl2 increased.
4) The lithium and lead contents of Mg-Li-Pb alloys can be controlled by changing MgCl2 and PbCl2 concentrations.
References
[1] CAILLERIE J L, ALBERT L. Lead-calcium alloy development: Quality improvement [J]. Journal of Power Sources, 1997, 67: 279-281.
[2] BUI N, MATTESCO P, SIMON P, STEINMETZ J, ROCCA E. The tin effect in lead-calcium alloys [J]. Journal of Power Sources, 1997, 67: 61-67.
[3] ABRAMOV V.O, SOMMER F, ORLOV D. Properties of Al-Pb base alloys applying electromagnetic forces and ultrasonic vibration during casting [J]. Materials Letters, 1995, 23: 17-20.
[4] DEMIRCI G, KARAKAYA ?. Collection of magnesium in an Mg-Pb alloy cathode placed at the bottom of the cell in MgCl2 electrolysis [J]. Journal of Alloys and Compounds, 2007, 439: 237-242.
[5] DEMIRCI G, KARAKAYA ?. Electrolytic magnesium production and its hydrodynamics by using an Mg-Pb alloy cathode [J]. Journal of Alloys and Compounds, 2008, 465: 255-260.
[6] BALASUBRAMANI N, SRINIVASAN A, PILLAIB U T S, PAI B C. Effect of Pb and Sb additions on the precipitation kinetics of AZ91 magnesium alloy [J]. Materials Science and Engineering A, 2007, A457: 275-281.
[7] MUKHOPADYAY I, SHARON M. Investigation of semiconducting parameters of Pb-Sn alloy oxide-electrolyte interface by Butler Gartner model [J]. Original Research Article solar Energy Materials and Solar Cells, 1997, 45: 141-149.
[8] LIN Zhen-han. Handbook of re?nement and metallurgy of nonferrous metal [M]. Beijing: Metallurgical Industry Press, 1999: 111-128. (in Chinese)
[9] POLYAKOVA L P, TAXIL P, POLYAKOV E G. Electrochemical behaviour and codeposition of titanium and niobium in chloride-?uoride melts [J]. Journal of Alloys and Compounds, 2003, 359: 244-255.
[10] YAN Y D, ZHANG M L, HAN W, CAO D X, YUAN Y, XUE Y, CHEN Z. Electrochemical formation of Mg–Li alloys at solid magnesium electrode from LiCl-KCl melts [J]. Electrochimica Acta, 2008, 53: 3323-3328.
[11] ZHANG M L, CHEN Z, HAN W, HOU Z Y, YAN Y D. Electrodeposition of Li and electrochemical formation of Mg-Li alloys from the eutectic LiCl-KCl [J]. Journal of Alloys and Compounds, 2008, 464: 174-178.
[12] CHEN Z, ZHANG M L, HAN W, WANG X L, TANG D X. Electrodeposition of Zr and electrochemical formation of Mg-Zr alloys from the eutectic LiCl-KCl [J]. Journal of Alloys and Compounds, 2008, 459: 209-214.
[13] YAN Y D, ZHANG M L, XUE Y, HAN W, CAO D X, WEI S Q. Study on the preparation of Mg-Li-Zn alloys by electrochemical codeposition from LiCl-KCl-MgCl2-ZnCl2 melts [J]. Electrochimica Acta, 2009, 54: 3387-3393.
[14] YAN Y D, ZHANG M L, XUE Y, HAN W, CAO D X, HE L Y. Electrochemical study of Mg–Li–Al alloys by codeposition from LiCl-KCl-MgCl2-AlCl3 melts [J]. Journal of Applied Electrochemistry, 2009, 39(3): 455-461.
[15] BERZINS T, DELAHAY P. Oscillographic polarographic waves for the reversible deposition of metals on solid [J]. Journal of the American Chemical Society, 1953, 75: 555-559.
[16] ?DEGARD R, BJ?RGUM A, STERTEN ?, THONSTAD J, TUNOLD R. Kinetics of aluminium deposition from aluminium chloride-alkali chloride melts [J]. Electrochemica Acta, 1982, 27(11): 1595-1598.
[17] BARD A J, FAULKNER L R. Electrochemical methods fundamentals and applications [M]. New York: John Wiley & Sons, 1980: 156-163.
[18] LAITY R W, MCINTYRE J D E. Chronopotentiometric diffusion coefficients in fused salts I. Theory [J]. Journal of the American Chemical Society, 1965, 87: 3806-3812.
[19] ZHANG Mi-lin, YAN Yong-de, HOU Zhi-yan, FAN Lu-an, CHEN Zeng, TANG Ding-xiang. Preparation of Mg-Li alloys by electrolysis in molten salt at low temperature[J]. Chinese Chemical Letters, 2007, 18: 329-332.
Pb(II)在LiCl-KCl-MgCl2-PbCl2熔盐体系中的电化学行为
张密林,陈丽军,韩 伟,颜永得,曹 鹏
哈尔滨工程大学 材料科学与化学工程学院,教育部超轻材料与表面技术重点实验室,哈尔滨 150001
摘 要:在LiCl-KCl-PbCl2-MgCl2熔盐体系中借助循环伏安和计时电位技术对Pb(II)的电化学行为以及Pb、Mg、Li的共沉积过程进行探讨,用不同的方法测算得到铅离子在熔盐中的扩散系数。循环伏安和计时电位的研究结果均表明,Li在先析出的Pb上发生欠电位沉积,生成液态的Li-Pb合金,而在熔盐中加入MgCl2后,会有相应的Mg-Li-Pb合金生成。用恒电流密度(6.21 A/cm2)电解2 h制备Mg-Li-Pb合金,并运用XRD对所得合金进行分析测 试。结果表明,在Mg-Li-Pb合金中存在β-Li、PbLi3、Mg2Pb等合金相,并可以通过控制熔盐中PbCl2和MgCl2的浓度来改变合金相的组成。
关键词:LiCl-KCl体系;循环伏安;计时电位;扩散系数;熔盐电解
(Edited by YANG Hua)
Foundation item: Projects (50871033, 21173060, 21103033) supported by the National Natural Science Foundation of China; Project supported by the Fundamental Research Funds for the Central Universities, China; Projects (2011AA03A409, 2009AA050702, 2007CB200906) supported by the Basic Research Foundation of Harbin Engineering University, China
Corresponding author: ZHANG Mi-lin; Tel: +86-451-82533026; E-mail: zhangmilin2010@sina.cn
DOI: 10.1016/S1003-6326(11)61235-1
Abstract: Cyclic voltammetry and chronopotentiometry were used to study the reaction mechanism of Pb(II) and the co-deposition of Pb, Mg and Li on molybdenum electrodes in LiCl-KCl-PbCl2-MgCl2 melts. The diffusion coefficient of lead ions in the melts was determined by different electrochemical techniques. The results obtained by cyclic voltammetry and chronopotentiometry indicated that the underpotential deposition of lithium on pre-deposited Pb leads to the formation of a liquid Li-Pb alloy, and the Mg-Li-Pb alloys are formed after the addition of MgCl2. X-ray diffraction confirmed that in the Mg-Li-Pb alloy, PbLi3, Mg2Pb and Li7Pb2 phases exist by galvanostatic electrolysis at 6.21 A/cm2 for 2 h at 873 K and the phases can be controlled by changing the concentration of PbCl2 and MgCl2.


