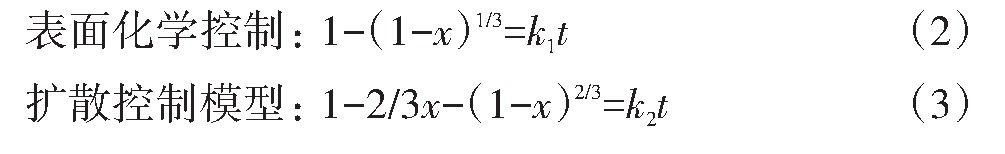网络首发时间: 2018-10-17 16:56
废旧锂离子电池正极材料LiNi0.5 Co0.2 Mn0.3 O2 中有价金属的浸出及其动力学研究
黄孝振 徐政 纪仲光 孙启
有研工程技术研究院有限公司生物冶金国家工程实验室
摘 要:
采用废旧锂离子电池正极材料LiNi0. 5Co0. 2Mn0. 3O2为原料,以H2SO4为浸出剂,H2O2为还原剂酸浸回收有价金属Li,Ni,Co,Mn;分别考察H2SO4浓度、H2O2浓度、固液比、浸出温度和浸出时间对浸出过程的影响,结果表明:在H2SO4浓度2. 5 mol·L-1、H2O23. 0%(原子分数)、固液比50 g·L-1、温度45℃、反应60 min的最佳条件下,Li,Ni,Co,Mn的浸出率均超过98. 5%。通过X射线衍射(XRD)和扫描电子显微镜和能量色散X射线谱(SEM-EDS)对不同浸出阶段的材料进行表征,可以得出废LiNi0. 5Co0. 2Mn0. 3O2在浸出过程中形貌和结构逐渐被破坏,在浸出终点衍射特征峰基本消失,表明废LiNi0. 5Co0. 2Mn0. 3O2被浸出完全,浸出渣只剩乙炔黑和黏结剂聚偏氟乙烯(PVDF)。采用未反应核收缩模型和Avrami方程模型对浸出动力学数据进行拟合,其中Avrami方程模型显示最佳相关性拟合,动力学分析显示,在30~75℃下Li,Ni,Co,Mn 4种金属离子的活化能分别为78. 39,81. 63,83. 07,82. 66 kJ·mol-1,表明浸出过程的速率控制步骤是表面化学反应。
关键词:
废旧锂离子电池 ;正极材料 ;LiNi0.5Co0.2Mn0.3O2 ;浸出动力学 ;
中图分类号: X705
作者简介: 黄孝振(1992-),男,河南信阳人,硕士研究生,研究方向:废旧锂离子电池的回收处理与资源化再生,E-mail:huangxz823@163.com;; *徐政,教授,电话:010-60662768,E-mail:xzh63@126.com;
收稿日期: 2018-06-10
基金: 国家国际科技合作专项项目(2014DFA90920)资助;
Leaching and Kinetics Study of Valuable Metals from LiNi0.5 Co0.2 Mn0.3 O2 Cathode in Spent LIBs
Huang Xiaozhen Xu Zheng Ji Zhongguang Sun Qi
National Engineering Laboratory of Biohydrometallurgy,GRINMAT Engineering Institute Co.,Ltd.
Abstract:
The cathode materialLiNi0.5 Co0.2 Mn0.3 O2 of the spent lithium batteries(LIBs)was used as the raw material,H2 SO4 was used as the leaching agent,and H2 O2 was used as the reducing agent to recover the valuable metals Li,Ni,Co,Mn by acid leaching. The effects of acid concentration,hydrogen peroxide concentration,solid to liquid ratio,leaching temperature and leaching time on the leaching rate of four valuable metals were investigated. The results showed that under the optimal conditions,such as the H2 SO4 concentration of 2.5 mol·L-1,H2 O2 of 3.0%(volume fraction),solid-liquid ratio of 50 g·L-1,temperature of 45 ℃,reaction time of 60 min,the leaching rates of Li,Ni,Co and Mn all exceeded 98.5%. The materials at different leaching stages were characterized by Xray diffraction(XRD)and scanning electron microscope-energy dispersive spectrometer(SEM-EDS),It could be concluded that the morphology and structure of the waste LiNi0.5 Co0.2 Mn0.3 O2 were gradually destroyed during the leaching process,the diffraction characteristic peaks basically disappear and only acetylene black and poly(vinylidene fluoride)(PVDF)remained in the leach residue. The unreacted nuclear contraction model and Avrami equation model were used to fit the leaching kinetics data,the Avrami equation model showed the best correlation fit. Kinetics analysis indicated that the data for the dissolution of Li,Ni,Co and Mn in the temperature range of 30~75 ℃ showed the best fit to the Avrami equation. The Eafor four metals were 78.39,81.63,83.07,82.66 kJ·mol-1,respectively. Kinetics analysis indicated that leaching process is controlled by surface chemical reaction.
Keyword:
spent lithium batteries(LIBs); cathode material; LiNi0.5Co0.2Mn0.3O2; leaching kinetics;
Received: 2018-06-10
锂离子电池具有能量密度高、放电率低等显著优点,被广泛于电子产品中,尤其是近年来在电动汽车上的大量应用,引发了对锂离子电池的强劲需求
[1 ]
。锂离子电池消费量增长的同时报废量也持续增加,废旧锂离子电池中含有丰富的Li,Co,Ni,Mn等有价金属和电解液等,对其资源化回收对缓解资源短缺和减轻环境污染具有重要意义
[2 ]
。
关于废旧锂离子电池资源化回收利用的技术,已有研究者进行了全面的总结
[3 ,4 ,5 ,6 ,7 ]
,其中,湿法冶金技术以其能耗低、有价金属回收率和纯度高等优点,是目前工艺化最成熟的方法
[8 ]
。湿法冶金的工艺中,浸出过程在整个回收过程中起着关键作用
[9 ]
,主要采用无机酸,如H2 SO4
[10 ]
、HNO3
[11 ]
、HCl
[12 ]
等,以及有机酸,如柠檬酸
[13 ]
、琥珀酸
[14 ]
等作浸出剂。
锂离子电池第一代正极材料是LiCoO2 ,由于在价格和能量密度等方面的限制,主要应用于消费类电子产品
[15 ]
,随着正极材料的研究突破,具有广阔前景的三元正极材料LiNix Coy Mnz O2 开始广泛用于消费类电子和电动汽车中
[16 ]
。目前,已成功商用的三元正极材料有LiNi1/3 Co1/3 Mn1/3 O2 和LiNi0.5 Co0.2 Mn0.3 O2 ,其中,LiNi0.5 Co0.2 Mn0.3 O2 在能量密度、价格、结构稳定性等方面具有综合优势已经成为动力电池的主流材料
[17 ]
。然而,目前废旧锂离子电池浸出研究主要集中在对LiCoO2 中Co和Li的浸出研究,对LiNix Coy Mnz O2 中Li,Ni,Co,Mn的浸出和动力学研究少有报道,且仅有少量LiNi1/3 Co1/3 Mn1/3 O2 的浸出和动力学研究,暂时没有关于LiNi0.5 Co0.2 Mn0.3 O2 研究的报道。对废旧锂离子电池三元正极材料LiNix Coy Mnz O2 的浸出行为和动力学研究,不仅对丰富相关理论数据有重要作用,更有助于提高此类浸出体系的浸出效率,为实际浸出提供指导。
正极材料通常与乙炔黑和黏结剂聚偏氟乙烯(PVDF)混合后涂布在铝箔上形成正极片。由于乙炔黑与PVDF不溶于酸,实际工业浸出是将与铝箔分离的混合物直接浸出。LiNi1/3 Co1/3 Mn1/3 O2 浸出和动力学研究的报道中,He等
[9 ]
直接将商业生产的LiNi1/3 Co1/3 Mn1/3 O2 作为浸出原料;Li等
[18 ,19 ]
为方便浸出研究,采用高温煅烧除去与废LiNi1/3 Co1/3 Mn1/3 O2 混合的乙炔黑和PVDF。上述研究均偏向于理论研究,脱离了废旧锂离子电池资源化回收的实际,无法为实际的浸出工艺提供较好的指导。
本研究采用报废的锂离子电池正极材料LiNi0.5 Co0.2 Mn0.3 O2 为原料,直接将与铝箔分离混合物研磨后对其中的Li,Ni,Co,Mn进行浸出研究,考察了不同条件对浸出过程的影响以获得最佳浸出条件,并对浸出过程进行动力学研究,为废旧锂离子电池三元类正极材料资源化回收和综合利用提供理论指导。
1 实验
1.1 材料与试剂
实验所用废旧锂离子电池为NCM523软包锂离子动力电池,由国联汽车动力电池研究院提供。先手工拆解得到正极片,再采用实验室发明的专有技术实现正极活性材料与铝箔的分离,正极活性材料经球磨后过0.15 mm标准筛得到废LiNi0.5 Co0.2 Mn0.3 O2 成分在90%左右的实验用混合粉料(以下简称粉料)。采用电感耦合等离子体发射光谱仪(ICP-OES)测得粉料的主要成分如表1所示。实验用H2 SO4 和30%(质量分数)的H2 O2 均为分析纯。
1.2 方法
称取5 g粉料置于250 ml的圆底烧瓶中,采用H2 SO4 溶液浸出并控制机械搅拌转速为500 r·min-1 ,考察H2 SO4 浓度、浸出时间、还原剂加入量、固液比和反应温度对浸出过程的影响。在特定时间取样并过滤,滤液采用ICP-OES测定Ni,Co,Mn,Li的含量,用式(1)计算各金属的浸出率(α,%)。
式中,C为浸出液中各金属的含量(g·ml-1 );V为浸出液的体积(ml);m为粉料的质量(g);ω为粉料中各金属元素的含量(%)。
粉料及浸出渣的形貌、结构用X射线衍射(XRD)、扫描电镜(SEM)和能谱分析仪(EDS)表征。
表1 粉料金属元素成分含量 下载原图
Table 1 Metal composition of powder(%,mass fraction)
2 结果与讨论
2.1 浸出条件对各金属浸出率的影响
2.1.1 H2SO4的浓度对浸出的影响
控制反应温度45℃、固液比50 g·L-1 、反应时间240 min,考察H2 SO4 浓度对各金属元素浸出率的影响,如图1所示。随着H2 SO4 浓度的增大,4种金属离子的浸出率逐渐升高,在酸浓度从1 mol·L-1 增加到2.5 mol·L-1 过程中,浸出曲线出现一个快速上升的阶段,此后的浸出率开始增长缓慢,综合考虑选择H2 SO4 最适宜浓度为2.5 mol·L-1 。由浸出曲线可以看出,Li的浸出率远高于其他3种金属离子,且Ni,Co,Mn的浸出率接近,这与其他研究
[20 ,21 ]
Ni的浸出率远高于Co,Mn的结果不同。这可能是因为本研究对象是三元正极废料,其中Li-O键的键长和结合能较低,很容易被酸液破坏,而Ni,Co,Mn 3种金属在三元正极材料α-NaFeO2 结构中结合能更高,因此更难被酸液破坏。
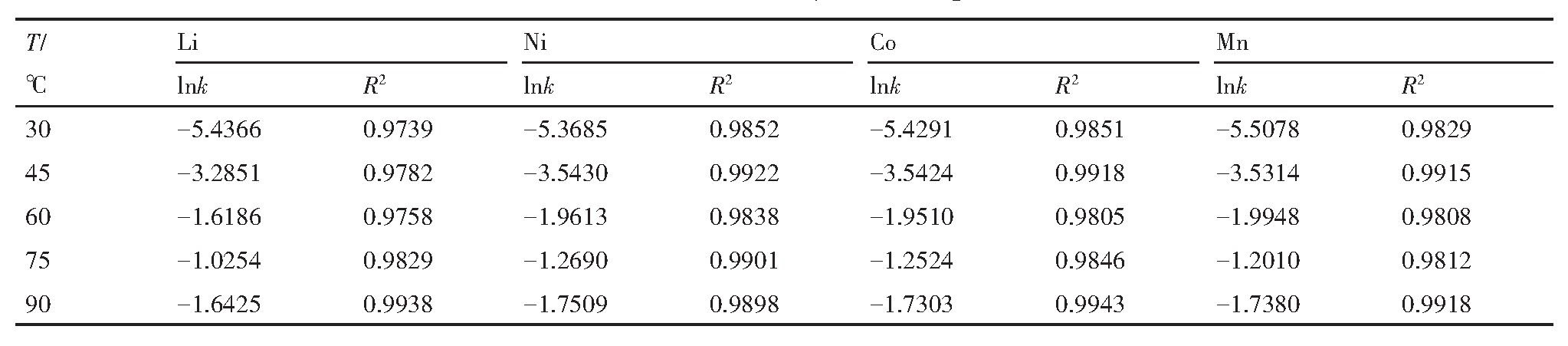
2.1.2 浸出时间对浸出过程的影响
H2 SO4 浓度2.5 mol·L-1 、反应温度45℃、固液比50 g·L-1 、浸出时间240 min的条件下,各金属元素浸出率变化曲线如图2所示。0~60 min内,4种金属离子的浸出率快速增加,60 min时Li,Ni,Co,Mn的浸出率分别为70.34%,35.13%,38.57%和36.25%,60 min后浸出曲线趋于平缓,浸出率提高不大。因此,后续实验浸出时间改为60 min更合适。
2.1.3 H2O2加入量对浸出的影响
LiNi0.5 Co0.2 Mn0.3 O2 材料中,Ni,Co,Mn的化合价分别为+2,+3,+4,Co3+ 和Mn4+ 较难浸出,需要使用H2 O2
[22 ]
,葡萄糖
[23 ]
,NaHSO3
[24 ]
等还原剂将Co3+ 和Mn4+ 还原成易溶的Co2+ 和Mn2+ ,从而提高浸出效率和浸出率
[25 ]
,其中,H2 O2 以其价格便宜,促进浸出效果显著,生成产物为H2 O不会对浸出液中有价金属回收产生影响等优点,使用最为普遍。
图1 H2SO4浓度对各金属浸出率的影响
Fig.1 Effect of acid concentration on leaching of different metals
图2 浸出时间对各金属浸出率的影响
Fig.2 Effect of leaching time on leaching of different metals
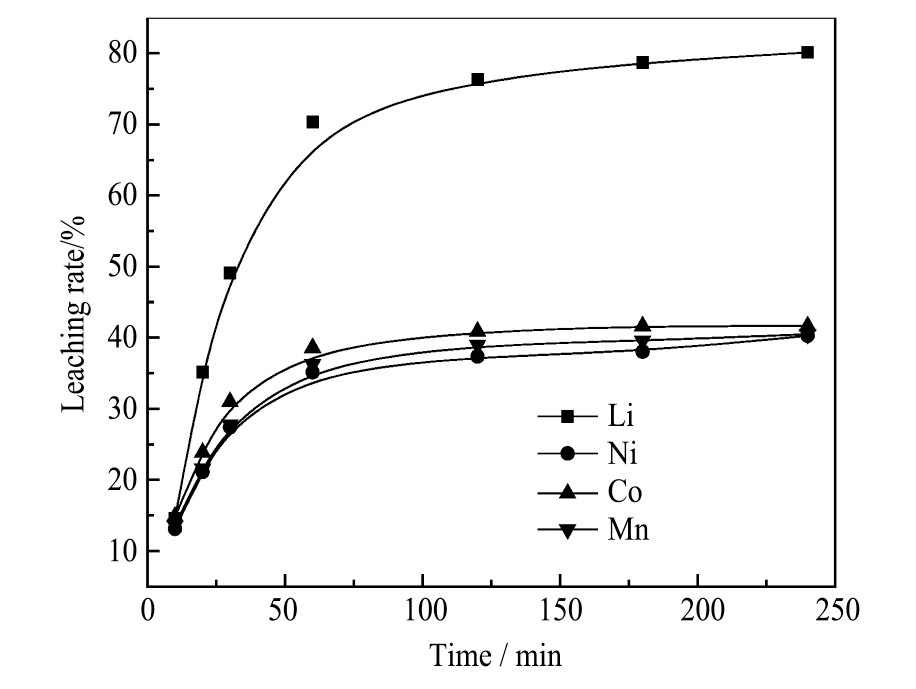
研究使用H2 O2 作还原剂,考察H2 O2 的加入量对各金属离子浸出率的影响。控制温度45℃、固液比50 g·L-1 、H2 SO4 浓度2.5 mol·L-1 、浸出时间60min,H2 O2 的加入量对各金属离子浸出率的影响如图3所示。可以看出,H2 O2 的加入使金属离子的浸出率显著提高,当H2 O2 的加入量为3.0%时,Li,Ni,Co,Mn的浸出率接近99%分别为98.60%,98.65%,98.70%,98.67%,而不加H2 O2 时上述金属离子浸出率仅为68.17%,33.83%,34.61%,33.62%。Co,Mn的浸出率显著提高,说明H2 O2 对Co3+ 和Mn4+ 有很好的还原效果,尽管Li和Ni的价态在浸出过程中没有变化,浸出率和浸出效率也显著提高,这可能是因为Co-O键和Mn-O键被破坏后,LiNix Coy Mnz O2 材料的结构被破坏,因此Li,Mn也更容易浸出
[26 ]
。H2 O2 的加入量增大至3.5%,各金属浸出率变化不大,因此H2 O2 的最佳加入量为3.0%。
图3 H2O2加入量对各金属浸出率的影响
Fig.3 Effect of amount of H2 O2 on leaching of different metals
2.1.4 反应固液比对浸出的影响
在H2 SO4 浓度2.5 mol·L-1 、温度45℃、浸出时间60 min、H2 O2 3.0%的条件下,考察固液比对浸出效果的影响,如图4所示。结果表明,固液比在30~50 g·L-1 的范围内,浸出曲线基本平衡,Li,Ni,Co,Mn的浸出率均在98%以上,当固液比超过50g·L-1 时,各金属的浸出率开始显著下降,这可能是因为较低固液比会增加粉料与浸出液的接触面积从而加速浸出反应
[27 ]
。综合考虑浸出效果与经济性以及浸出液浓度的提高有利于后续有价金属的回收处理,最佳固液比确定为50 g·L-1 。
2.1.5 反应温度对浸出的影响
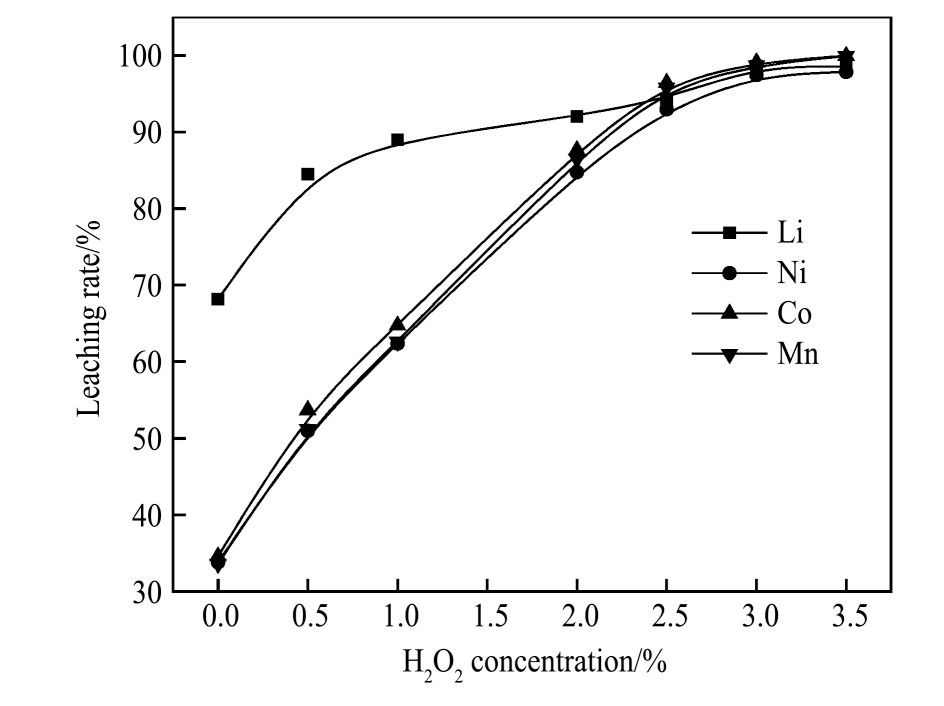
反应温度与浸出反应活化能密切相关,因此在H2 SO4 2.5 mol·L-1 、固液比50 g·L-1 、浸出时间60 min、H2 O2 3.0%的条件下,考察温度对浸出效果的影响,如图5所示。反应温度在30℃时,Li,Ni,Co,Mn的浸出率分别为90.98%,87.08%,87.94%,89.88%,当温度上升到45℃时,四种金属离子的浸出率明显提高,均在98.5%以上。此后,随着温度继续提高,浸出曲线略有浮动但浸出率提高不大且温度在90℃时各金属浸出率有所降低。因此,选择温度为45℃时即可实现各金属浸出率均大于98.5%的显著效果。
2.2 结构表征及动力学分析
2.2.1 结构表征
图4 反应固液比对各金属浸出率的影响
Fig.4 Effect of pulp density on leaching of different metals
图5 反应温度对各金属浸出率的影响
Fig.5 Effect of temperature on leaching of different metals
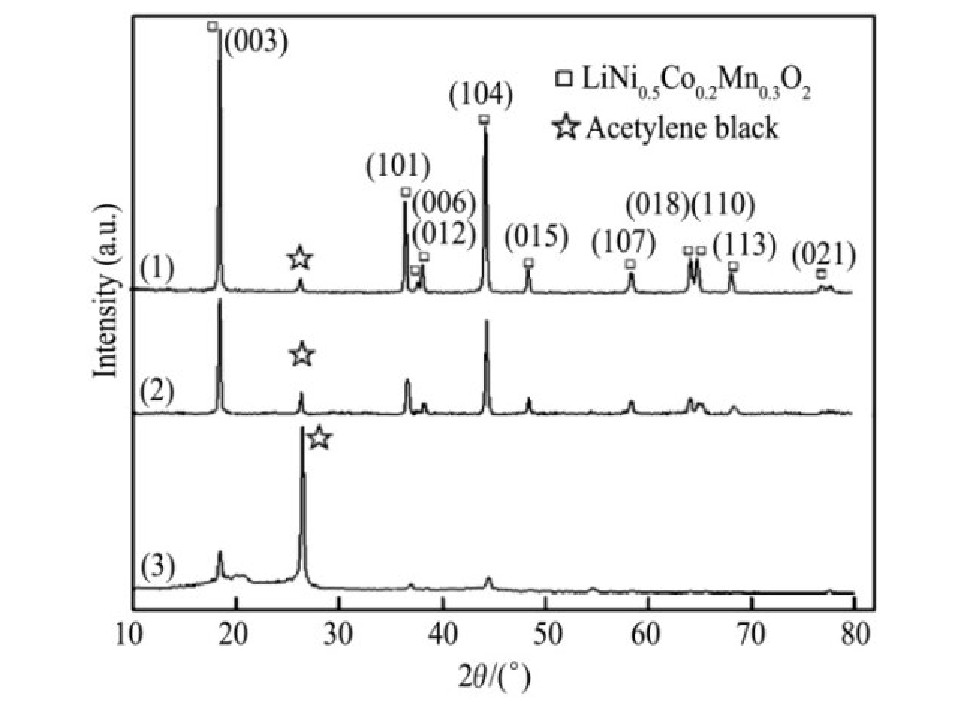
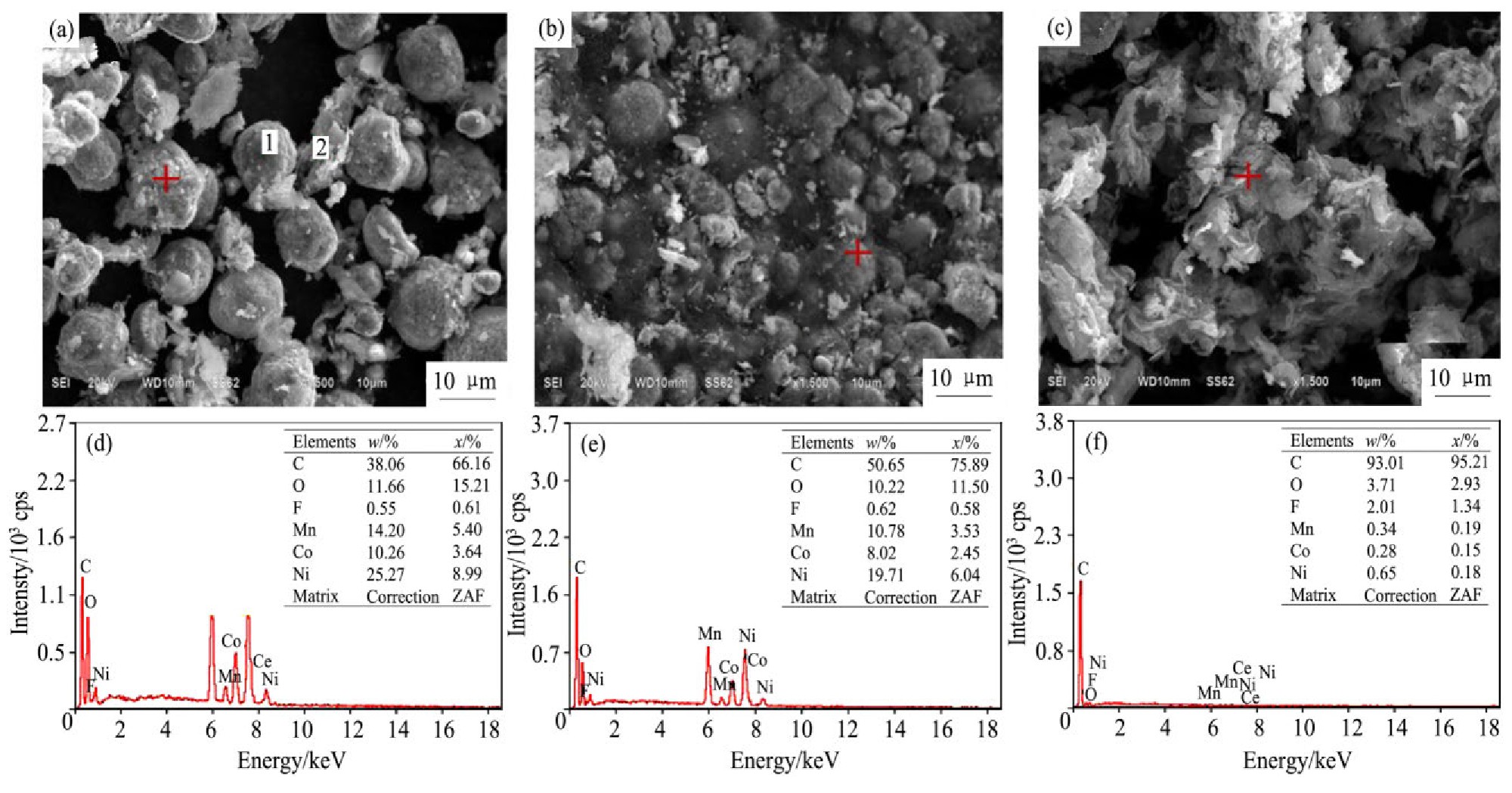
为了更直观地反映粉料中废LiNi0.5 Co0.2 Mn0.3 O2 在酸浸过程中的结构、形貌的变化。对浸出前的粉料与浸出5,60 min后的浸出渣(H2 SO4 浓度2.5 mol·L-1 ,H2 O2 3.0%、固液比50 g·L-1 、温度45℃最佳条件下),这3种固体进行XRD和SEM-EDS分析,结果分别如图6和7所示。
由图6可以看出,浸出前粉料的XRD图谱显示出LiNi0.5 Co0.2 Mn0.3 O2 材料良好的特征峰,浸出5min后的浸出渣对应特征峰开始减弱或略有偏移,这说明LiNi0.5 Co0.2 Mn0.3 O2 材料的晶体结构在酸浸过程中被破坏
[9 ]
,60 min后的浸出渣XRD图谱仅有少数对应的微弱特征峰。上述3个固体的XRD图谱中均出现了乙炔黑的衍射峰,且峰强依次增大。
图7(a)为浸出前粉料的SEM图,可以看出,废LiNi0.5 Co0.2 Mn0.3 O2 依然保持良好的球形颗粒,乙炔黑和PVDF黏结剂成不规则形状,经研磨后大部分与废LiNi0.5 Co0.2 Mn0.3 O2 解离,少量黏结在球形颗粒上,对图7(a)上的多点进行EDS分析,选取图上标记的点结果如图7(d)所示,Ni,Co,Mn的含量与ICP-OES测得的结果基本一致。图7(b)为浸出5min后浸出渣的SEM图,可以看出球形颗粒基本被破坏,这与对应的XRD图谱的特征峰减弱或略有偏移相一致。图7(c)为浸出60 min后浸出渣的SEM图,选取对应点的EDS分析如图7(f)所示,可以看出浸出渣的主要成分为C,F等,说明此时浸出渣主要为不溶的乙炔黑和PVDF,Ni,Co,Mn含量低于0.7%,这与各金属离子浸出率均在98.5%以上相对应。
图6 浸出前粉料、5 min后浸出渣,60 min后浸出渣的XRD图谱
Fig.6 XRD patterns of cathode material powder(1),leach-ing residue at 5 min(2),leaching residue at 60 min(3)
图7 浸出前粉料,5 min后浸出渣和60 min后浸出渣的SEM-EDS分析
Fig.7 SEM-EDS analysis of(a,d)cathode material powder,(b,e)leaching residue at 5 min,(c,f)leaching residue at 60 min
2.2.2 动力学分析
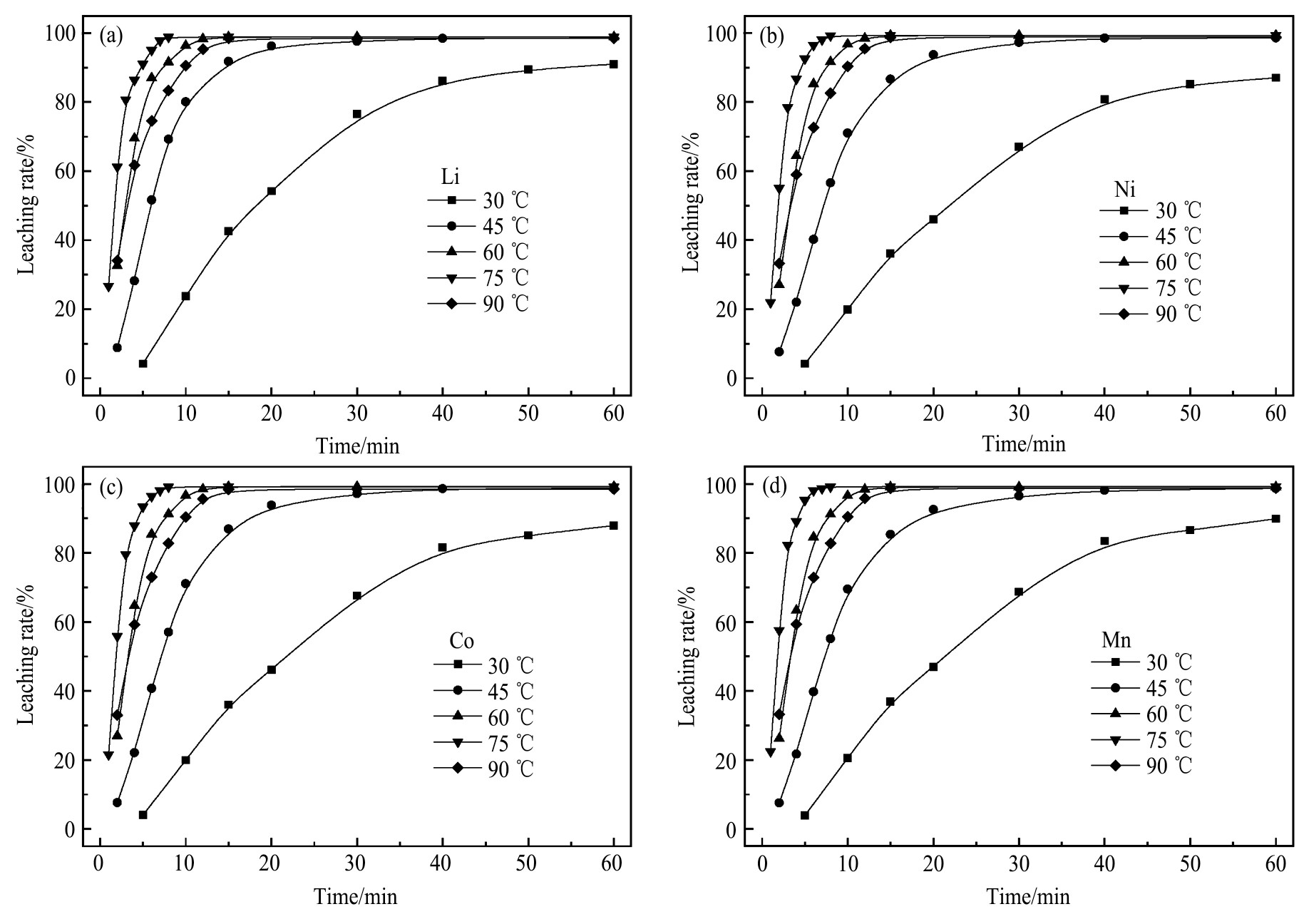
为了揭示酸浸过程中可能的浸出机制,保持在H2 SO4 2.5 mol·L-1 、固液比50g·L-1 、H2 O2 3.0%参数不变的条件下,对不同温度下各金属离子的浸出率随时间变化作浸出动力学曲线,如图8所示。可以看出,Li,Ni,Co,Mn的浸出率随着浸出时间的增加不断增大,且前期浸出效率明显大于后期,浸出曲线在后期趋于平衡。当温度由30℃提高到75℃时,浸出平衡时间由60min减少到8 min。文献
[
28 ,
29 ,
30 ]
报道,LiCoO2 的浸出平衡时间均在120 min以上,这说明LiNi0.5 Co0.2 Mn0.3 O2 的浸出动力学明显快于LiCoO2 。当浸出温度为90℃时,4种金属离子的浸出曲线均在60℃和75℃时浸出曲线的下方,表明90℃时4种金属离子的浸出速率相对开始减小,且由图8可以看出浸出平衡时间也增至15 min。经过分析,出现这种情况的原因可能是由于H2 O2 易于分解,在70℃以上时,分解速率加快
[31 ]
,当浸出温度为90℃时,H2 O2 的分解量增大,此时浸出体系中的H2 O2 相对减少,因此浸出速率相对开始减小。
浸出的粉料由可溶的废LiNi0.5 Co0.2 Mn0.3 O2 和不溶的乙炔黑和PVDF组成。在反应过程中,LiNi0.5 Co0.2 Mn0.3 O2 不断浸出,反应界面不断向内收缩,残余的固体物质形成疏松多孔的灰层,动力学符合未反应收缩核模型。该模型又分为以下3种类型:(1)气膜扩散控制;(2)灰层扩散控制;(3)表面化学反应控制。浸出过程中,废LiNi0.5 Co0.2 Mn0.3 O2 不断溶解且没有固相生成,可认为是结晶的逆过程,因此,Avrami方程也适用于该反应动力学过程
[32 ,33 ]
。
上述4种动力学模型方程如下:
图8 锂离子电池正极废料LiNi0.5Co0.2Mn0.3O2浸出动力学曲线
Fig.8 Leaching kinetics curves of spent LiNi0.5 Co0.2 Mn0.3 O2 material
(a)Li;(b)Ni;(c)Co;(d)Mn
式中,x是各金属离子浸出率;k是反应速率常数;t是浸出时间;n是线性拟合的合适的参数。
采用4种动力学模型对浸出动力学数据进行拟合,其中,Avrami方程模型R2 均大于0.98显示浸出过程的最佳相关性拟合效果。
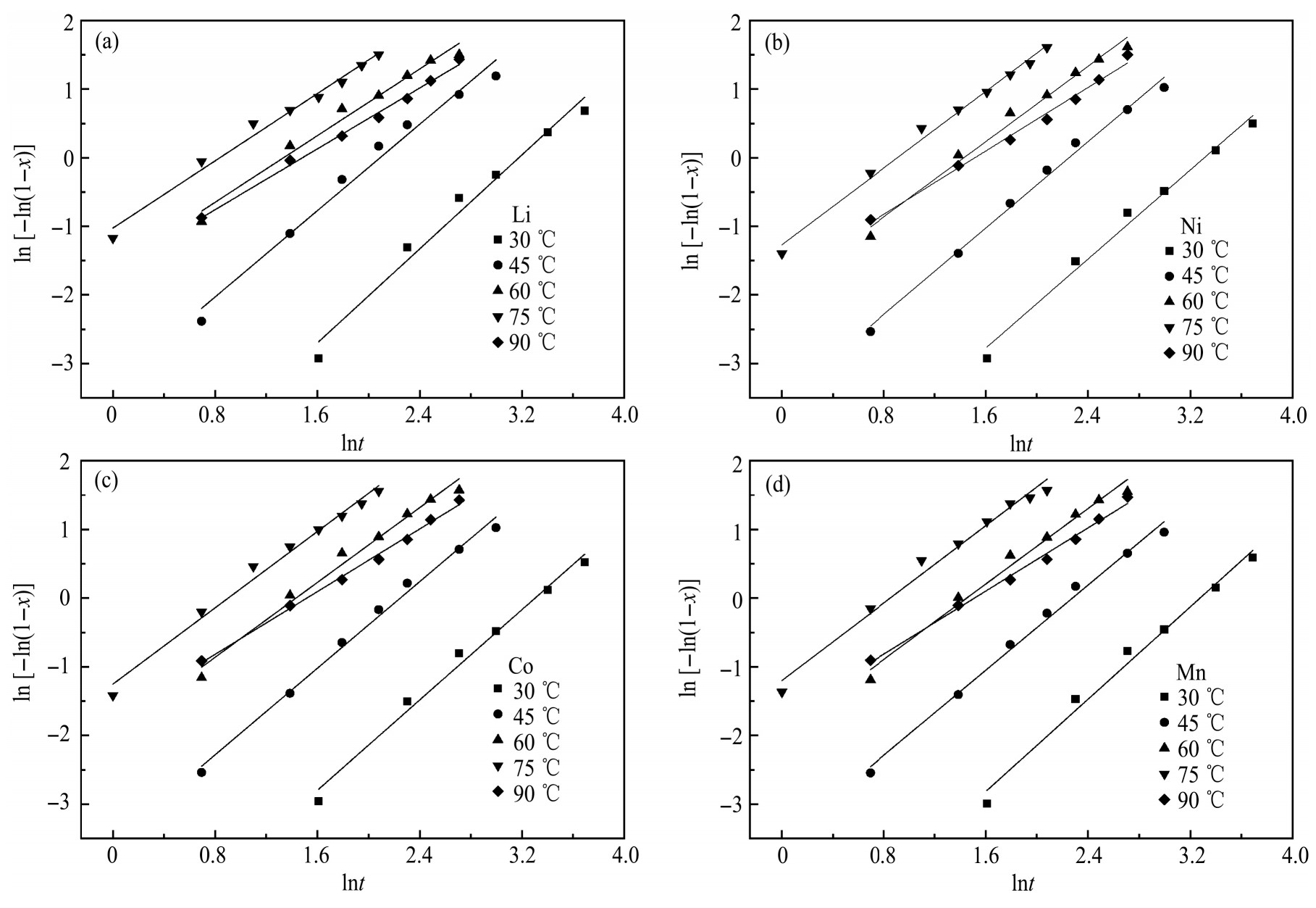
采用Avrami方程模型对4种金属的浸出动力学数据作ln[-ln(1-x)]与lnt拟合曲线,结果如图9和表2所示。4种金属在不同温度下的拟合系数R2 均大于0.97,说明Avrami方程模型能很好地反应整个浸出过程。斜率n的范围在1.10~1.72之间,表明浸出反应的初始速率很高,并随着浸出时间的增大速率不断降低,直至最后反应趋于平衡。随着温度的升高(30~75℃),lnk不断增大,说明温度能显著改变浸出反应的速率。
为了明确浸出的控制步骤,采用Arrhenius方程计算浸出反应的表观活化能
[34 ]
。
式中,k(min-1 )为反应速率常数;A为频率因子;Ea 为表观活化能;R为气体常数;T(K)为绝对温度。浸出反应的活化能可以用Arrhenius方程的线性形式:
表2 Avrami方程拟合Li,Ni,Co,Mn的动力学参数 下载原图
Table 2 Parameters of kinetics for Li,Ni,Co,and Mn by using Avrami equation
图9 不同反应温度下ln[-ln(1-x)]与lnt的关系
Fig.9 Plots of ln[-ln(1-x)]vs.lnt under different reaction temperature
(a)Li;(b)Ni;(c)Co;(d)Mn
以表2中30~75℃的lnk为纵坐标,T-1 为横坐标对Arrhenius方程拟合,求各金属离子浸出反应的活化能(Ea) ,结果如图10所示。从废旧锂离子电池正极材料LiNi0.5 Co0.2 Mn0.3 O2 中浸出Li,Ni,Co,Mn四种金属离子的活化能分别为78.39,81.63,83.07,82.66 kJ·mol-1 ,均大于40 kJ·mol-1 ,表明浸出过程的速率控制步骤是表面化学反应
[35 ]
。浸出反应所需的活化能越高,浸出反应越困难。因此,在浸出LiNi0.5 Co0.2 Mn0.3 O2 的反应中,Li最易被浸出,Co最难被浸出。
3 结论
采用还原酸浸法研究了废旧锂离子电池正极材料LiNi0.5 Co0.2 Mn0.3 O2 中有价金属的浸出及动力学,得到以下结论:
1.在H2 SO4 浓度2.5 mol·L-1 ,H2 O2 用量3.0%,固液比50 g·L-1 、温度45℃、反应时间60 min的最佳条件下,Li,Ni,Co,Mn的浸出率分别为均在98.5%以上。
2.对浸出前粉料和浸出渣采用XRD和SEM-EDS进行结构和形貌的表征,反映了废LiNi0.5 Co0.2 Mn0.3 O2 由饱满的球形颗粒到球形颗粒被破坏最后浸出渣中基本只剩不溶的乙炔黑和PVDF的过程。
3.LiNi0.5 Co0.2 Mn0.3 O2 的还原酸浸过程符合Avrami方程模型,浸出Li,Ni,Co,Mn的表观活化能分别为78.39,81.63,83.07,82.66 kJ·mol-1 ,表面化学反应是浸出过程的速率控制步骤。
图1 0 Li,Ni,Co和Mn浸出反应的Arrhenius拟合曲线
Fig.10 Arrhenius plots for the leaching of Li(a),Ni(b),Co(c),Mn(d)from LiNi0.5 Co0.2 Mn0.3 O2
参考文献
[1] Zheng X,Gao W,Zhang X,He M,Lin X,Cao H,Zhang Y,Sun Z. Spent lithium-ion battery recycling reductive ammonia leaching of metals from cathode scrap by sodium sulphite[J]. Waste Management,2017,60:680.
[2] Gao W,Liu C,Cao H,Zheng X,Lin X,Wang H.Comprehensive evaluation on effective leaching of critical metals from spent lithium-ion batteries[J]. Waste Management,2018,75:477.
[3] Zhang W,Xu C,He W,Li G,Huang J. A review on management of spent lithium ion batteries and strategy for resource recycling of all components from them[J].Waste Management&Research,2018,36(2):99.
[4] Ordo?ez J,Gago E J,Girard A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries[J]. Renewable and Sustainable Energy Reviews,2016,60:195.
[5] Lv W,Wang Z,Cao H,Sun Y,Zhang Y,Sun Z. A critical review and analysis on the recycling of spent lithium-ion batteries[J]. Acs Sustainable Chemistry&Engineering,2018,6(2):1504.
[6] Wu Y,Pei F,Jia L L,Liu X L,Zhang W H,Liu P.Overview of recovery technique of valuable metals from spent lithium ion batteries[J]. Chinese Journal of Rare Metals,2013,37(2):320.(吴越,裴锋,贾蕗路,刘晓磊,张文华,刘平.废旧锂离子电池中有价金属的回收技术进展[J].稀有金属,2013,37(2):320.)
[7] Li J B,Xu Z,Ji Z G,Sun Q,Huang X Z. An overview on the current technologies of recycling spent lithiumion batteries[J]. Chinese Journal of Rare Metals,2019,43(2):201.(李建波,徐政,纪仲光,孙启,黄孝振.废旧锂离子动力电池回收的研究现状[J].稀有金属,2019,43(2):201.)
[8] Zhang X,Xie Y,Lin X,Li H,Cao H. An overview on the processes and technologies for recycling cathodic active materials from spent lithium-ion batteries[J]. Journal of Material Cycles and Waste Management,2013,15(4):420.
[9] He L,Sun S,Song X,Yu J. Leaching process for recovering valuable metals from the LiNi1/3 Co1/3 Mn1/3 O2 cathode of lithium-ion batteries[J]. Waste Management,2017,64:171.
[10] Pagnanelli F,Moscardini E,Altimari P,Abo Atia T,Toro L. Leaching of electrodic powders from lithium ion batteries:optimization of operating conditions and effect of physical pretreatment for waste fraction retrieval[J].Waste Management,2017,60:706.
[11] Li Y,Xi G,Xi Y. Recovery of Co,Mn,Ni,and Li from spent lithium ion batteries for the preparation of LiNix Coy Mnz O2 cathode materials[J]. Ceramics International,2015,41(9):11498.
[12] JouliéM,Laucournet R,Billy E. Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries[J]. Journal of Power Sources, 2014,247:551.
[13] Santana I L,Moreira T F M,Lelis M F F,Freitas M B.Photocatalytic properties of Co3 O4 /LiCoO2 recycled from spent lithium-ion batteries using citric acid as leaching agent[J]. Materials Chemistry and Physics,2017,190:38.
[14] Li L,Qu W,Zhang X,Lu J,Chen R,Wu F,Amine K. Succinic acid-based leaching system:A sustainable process for recovery of valuable metals from spent Li-ion batteries[J]. Journal of Power Sources, 2015,282:544.
[15] He P,Yu H,Li D,Zhou H. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries[J]. Journal of Materials Chemistry,2012,22(9):3680.
[16] Tan T Q,Idris M S,Osman R A M,ReddyM V,ChowdariB V R. Structure and electrochemical behaviour of LiNi0. 4 Mn0. 4 Co0. 2 O2 as cathode material for lithium ion batteries[J]. Solid State Ionics,2015,278:43.
[17] Kong J,Zhai H,Ren C,Gao M,Zhang X,Li H,Li J,Tang Z,Zhou F. Synthesis and electrochemical performance of macroporous LiNi0. 5 Co0. 2 Mn0. 3 O2 by a modified sol-gel method[J]. Journal of Alloys and Compounds,2013,577:507.
[18] Li L,Bian Y,Zhang X,Guan Y,Fan E S,Wu F,Chen R J. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching[J]. Waste Management,2018,71:362.
[19] Meng Q,Zhang Y,Dong P,Liang F. A novel process for leaching of metals from LiNi1/3 Co1/3 Mn1/3 O2 material of spent lithium ion batteries:Process optimization and kinetics aspects[J]. Journal of Industrial and Engineering Chemistry,2018,61:133.
[20] Meshram P,Pandey B D,Mankhand T R. Hydrometallurgical processing of spent lithium ion batteries(LIBs)in the presence of a reducing agent with emphasis on kinetics of leaching[J]. Chemical Engineering Journal,2015,281:418.
[21] Meshram P,Pandey B D,Mankhand T R. Recovery of valuable metals from cathodic active material of spent lithium ion batteries:leaching and kinetic aspects[J].Waste Management,2015,45:306.
[22] Gao W,Liu C,Cao H,Zheng X,Lin X,Wang H,Zhang Y,Sun Z. Comprehensive evaluation on effective leaching of critical metals from spent lithium-ion batteries[J]. Waste Management,2018,75:477.
[23] Meng Q,Zhang Y,Dong P. Use of glucose as reductant to recover Co from spent lithium ions batteries[J].Waste Management,2017,64:214.
[24] Meshram P,Abhilash,Pandey B D,Mankhand T R,Jom H D. Comparision of different reductants in leaching of spent lithium ion batteries[J]. JOM,2016,68(10):2613.
[25] Zhang X,Cao H,Xie Y,Ning P,An H,You H,Nawaz F. A closed-loop process for recycling LiNi1/3 Co1/3 Mn1/3 O2 from the cathode scraps of lithium-ion batteries:process optimization and kinetics analysis[J]. Separation and Purification Technology,2015,150:186.
[26] Li L,Ge J,Chen R,WuF,Chen S,Zhang X X. Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries[J]. Waste Management,2010,30(12):2615.
[27] Zheng X,Gao W,Zhang X,He M,Lin X,Cao H,Zhang Y,Sun Z. Spent lithium-ion battery recyclingReductive ammonia leaching of metals from cathode scrap by sodium sulphite[J]. Waste Management,2017,60:680.
[28] Zhang Y,Meng Q,Dong P,Duan J,Lin Y. Use of grape seed as reductant for leaching of cobalt from spent lithium-ion batteries[J]. Journal of Industrial&Engineering Chemistry,2018,66:86.
[29] Zhang J,Man R L,Xu X Q,He F,Wu Q,Yin X Y.Thermodynamic and kinetic of elec-trolytic leaching cobalt from waste lithium-ion battery[J]. The Chinese Journal of Nonferrous Metals,2014,(4):993.(张建,满瑞林,徐筱群,贺凤,吴奇,尹晓莹.电解浸出废旧锂电池中钴的热力学和动力学[J].中国有色金属学报,2014,(4):993.)
[30] He F,Man R L,Liu Q,Sun Z M,Xu J,Zhang J. Kinetics of acid leaching cobalt from waste lithium-ion batteries using oat straw[J]. The Chinese Journal of Nonferrous Metals,2015,(4):1103.(贺凤,满瑞林,刘琦,孙祖眉,徐娟,张建.燕麦秸秆酸浸回收废旧锂电池中Co的动力学[J].中国有色金属学报,2015,(4):1103.)
[31] Xu Z Z,Li X C. Hydrogen peroxide decomposition effect factor analysis[J]. Textile Dyeing and Finishing,2006,28(1):33.(许志忠,李晓春.过氧化氢分解影响因素分析[J].染整技术,2006,28(1):33.)
[32] Soki?M D,Markovi?B,?ivkovi?D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid[J].Hydrometallurgy,2009,95(3-4):273.
[33] Li G,Rao M,Jiang T,Huang Q,Peng Z. Leaching of limonitic laterite ore by acidic thiosulfate solution[J].Minerals Engineering,2011,24(8):859.
[34] Golmohammadzadeh R,Rashchi F,Vahidi E. Recovery of lithium and cobalt from spent lithium-ion batteries using organic acids:process optimization and kinetic aspects[J]. Waste Management,2017,64:244.
[35] Rendón-Angeles J C,Matamoros-Veloza Z,Matamoros Veloza A,Perez-GaribayR,Rodriguez-Galicia J,Kazumichi Y. Facile synthesis of perovskite-structured powders using Barite-Celestite ore under hydrothermal alkaline conditions[J]. Industrial&Engineering Chemistry Research,2017,56(36):99422.