
Behavior of silicon-containing minerals during Bayer digestion
ZHAO Qing-jie(赵清杰)1, 2, YANG Qiao-fang(杨巧芳)1, CHEN Qi-yuan(陈启元)2,
YIN Zhou-lan(尹周澜)2, WU Zheng-ping(吴争平)2, YIN Zhen-guo(殷振国)3
1. Zhengzhou Research Institute, Aluminum Corporation of China Limited, Zhengzhou 450041, China;
2. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
3. Shenzhen Herewin Technology Co.,Ltd, Shenzhen 518140, China
Received 6 July 2009; accepted 30 December 2009
____________________________________________________________________________________________
Abstract:
Digestion behaviors of several single silicon-containing minerals including kaolinite, illite and pyrophyllite in aluminate solution were investigated. The result shows that the digestion rates of kaolinite, illite and pyrophyllite in aluminate solution were influenced by reaction conditions such as temperature. Adopting quantum chemistry theoretical calculation, using CASTEM procedure module, the slab model of kaolinite, illite, pyrophyllite and their ordinary complete cleavage plane (001) were geometry optimized at GGA-PW91 level. The micro-essence of the different reaction activities among kaolinite, illite, pyrophyllite in aluminate solution was opened out. It is found that the reaction activities of kaolinite, illite, pyrophyllite are obviously different and the reaction activity of pyrophyllite is the highest. The Si—O bond strength of illite is the strongest and its Al—O bond strength is comparatively the weakest, so Si in it is the most difficult to be removed. The Si—O bond strength of pyrophyllite is the weakest, so Si in it is relatively easy to be removed. The micro-electron structure of complete cleavage plane and the Si—O bond strength of kaolinite, illite and pyrophyllite are evidently influenced by the existence of OH-, of which the surface character and the reduction of Si—O bond strength of kaolinite are influenced most greatly by OH-. Therefore, the desilication of kaolinite in alkaline solution at relatively low temperature is easier.
Key words:
silicon minerals; alumina; Bayer digestion; aluminate solution; reaction activity; behavior;
____________________________________________________________________________________________
1 Introduction
Silicon-containing minerals are the general impurities in bauxite including kaolinite, illite, pyrophyllite etc. They are decomposed by alkali solution and enter into the solution in the form of Na2SiO3 during digestion, which is the key process of Bayer process [1-3]. Then, the Na2SiO3 reacts with aluminate solution, forming sodium aluminosilicate hydrate, most of which enters into the red mud and a little of which remains in the solution and deposits slowly. The silicon in the solution will result in a series of harms, such as the loss of alumina, the decrease of product quality and the formation of scar on facilities and pipes[4-9].
Because of the harms of silicon-containing minerals to alumina production, many studies based on the behavior of silicon-containing minerals in alumina process have been done. Some researchers studied the behavior of kaolinite in bauxite during digestion[9-11] and found that the desilication ratio was 20%-90% on the conditions of Na2Ok concentration 200-240 g/L, solid content 10%, temperature 98 ℃ and desilication time 9 h. Studies[5-6, 9, 12-13] on the behavior of illite in bauxite show that little illite reacts when temperature is lower than 150 ℃ and at 180 ℃ the reaction rate is slow. The reaction rate increases quickly when temperature is higher than 200 ℃. Study on the reaction between pyrophyllite and alkali solution has less been reported in any literature. The silicon- containing minerals whose behavior was illustrated by most researchers exist in the bauxite. The behavior of single silicon-containing minerals has less been studied by now. Furthermore, the change of the reaction rate between silicon minerals and alkali solution has less been analyzed theoretically in detail. In this work, the behavior of several single silicon-containing minerals such as kaolinite, illite, pyrophyllite in aluminate solution was investigated and the microcosmic reaction mechanism of kaolinite, illite, pyrophyllite in aluminate solution was discussed using quantum chemistry calculation.
2 Behavior of kaolinite during digestion
The structure unit of kaolinite belongs to double layer type (Toa type), that is, a [SiO4] tetrahedron connects with a [AlO2(OH)4] octahedron to form unit layer. The structure units are linked by hydrogen oxygen—hydrogen bond and the chemical bond between the layers is hydrogen bond. The structure of kaolinite is accumulated by structure unit. The difficulty of the removal of kaolinite in aluminate solution changes with its different forms in the bauxite.
2.1 Materials
The material used in the experiments is kaolinite ore with high purity. The average particle size of kaolinite is 31.13 μm. The aluminate solution is prepared by NaOH (AR) and industrial Al(OH)3.
2.2 Methodology
Experiments were carried out in steel bomb reactor with rotation speed of 36 r/min. 20 g kaolinite and 50 mL aluminate solution were added into the steel bomb to form slurry, and then the steel bomb was put into the reactor for the digestion to occur. The reaction rate of SiO2 in kaolinite is calculated by the quality of dissoluble SiO2 divided by the reaction time
2.3 Behavior of kaolinite in aluminate solution
Fig.1 shows the change of reaction rate of kaolinite in sodium aluminate solution with different reaction time under the conditions of 95 ℃, Na2Ok concentration 230 g/L and αk 3.0. Fig.2 shows the change of reaction rate of kaolinite in different alkali solutions with the same Na2Ok concentration.
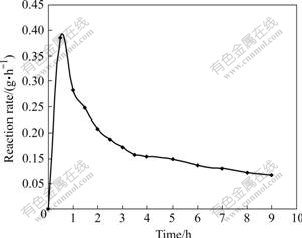
Fig.1 Reaction rate of kaolinite in sodium aluminate solution
Fig.1 and Fig.2 demonstrate that the reaction rate of kaolinite decreases with the increase of reaction time in different alkali solutions. The figures also indicate that the concentration of dissociated OH- in the solution is one of the most important factors on the dissolution ratio of kaolinite. Therefore, the increase of the concentration of OH- in the solution can speed up the dissolution of kaolinite.
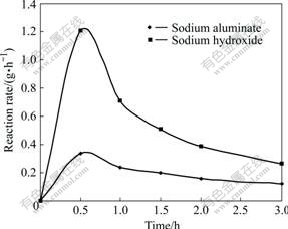
Fig.2 Change of reaction rate of kaolinite in different alkali solutions
Fig.3 and 4 show the different reaction rate of kaolinite in sodium aluminate solution at different reaction temperatures. The figures indicate that the raise of the reaction temperature can increase the dissolution ratio of kaolinite greatly.
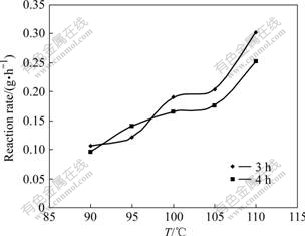
Fig.3 Reaction rate of kaolinite in sodium aluminate solution at different temperatures
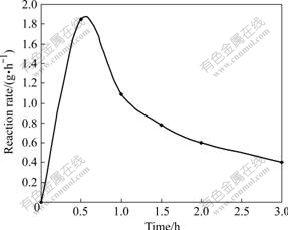
Fig.4 Change of reaction rate with time at 120 ℃
3 Behavior of illite during digestion
Illite belongs to interlayer cation deficient micas with dioctahedral structure. The structure unit of illite is three layer type (ToaT type) which consists of two [(SiAl)O4] layer and one octahedron layer. Both the electrovalence in the structure unit layer and that between the structure unite layers are unbalanced. During the digestion, illite is decomposed at first by alkali and enters into the solution as sodium silicate, then the sodium silicate reacts with sodium aluminate solution to form sodium aluminosilicate hydrate, which is silicon slag. Previous researches[10-11] are mainly concerned with the illite in bauxite.
3.1 Materials
The material used in the experiments is illite ore with high purity. The average particle size of illite is 40.0 μm. The aluminate solution is prepared by NaOH (AR) and industrial Al(OH)3.
3.2 Methodology
Methodology is the same as 2.2. The reaction rate of SiO2 in illite is calculated by the quality of dissoluble SiO2 divided by the reaction time.
3.3 Behavior of illite in sodium aluminate solution
Fig.5 shows the change of reaction rate of illite in sodium aluminate solution with different Na2Ok concentrations(150-230 g/L) under the conditions of 95 ℃, reaction time 5 min and αk 3.0. Fig.6 shows the change of reaction rate of illite in aluminate solution at different temperatures with the same Na2Ok concentration.
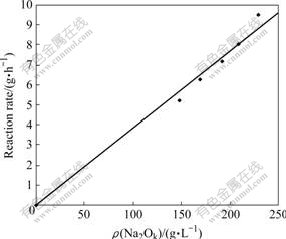
Fig.5 Effect of alkali concentration on reaction rate of illite
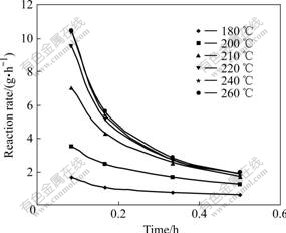
Fig.6 Change of reaction rate of illite with time at different temperatures
Fig.5 shows that the concentration of Na2Ok has great influence on reaction rate of illite and the dissolution rate increases with the increase of concentration. Fig.6 shows that reaction rate of illite decreases gradually with the increase of time. By drawing a graph of lnK against 1/T according to the data processing method in Ref.[14], the line slope -E/R can be obtained to be -13.8. Then, the activation energy E of the reaction of illite with sodium aluminate solution can be calculated to be 114.73 kJ/mol, which indicates that the reaction of illite in sodium aluminate solution is controlled by chemical reaction.
Fig.7 shows the change of reaction rate of illite with different average particle sizes of 40.00 μm and 2.35 μm in sodium aluminate solution under the conditions of 230 ℃, Na2Ok concentration 230 g/L and αk 3.0. It is indicated that particle size has little influence on the reaction rate of illite.
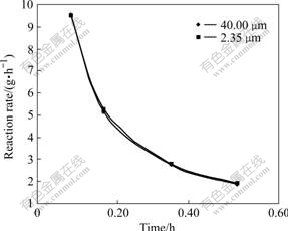
Fig.7 Reaction rate of illite with different particle sizes
4 Behavior of pyrophyllite during digestion
The structure unit of pyrophyllite belongs to three layer type (ToaT type), that is a [SiO4] tetrahedron and a [AlO2(OH)4] octahedron connect with a [SiO4] tetrahedron to form the unit layer, which is of dioctahedral structure[15].
4.1 Materials
The material used in the experiments is high purity pyrophyllite containing a small quantity of quartz etc. The average particle size of pyrophyllite is 5.08 μm. The sodium aluminate solution is prepared by NaOH (AR) and industrial Al(OH)3.
4.2 Methodology
Methodology is the same as section 2.2. The reaction rate of SiO2 in pyrophyllite is calculated by the quality of dissoluble SiO2 divided by the reaction time.
4.3 Behavior of pyrophyllite in sodium aluminate solution
Figs.8 and 9 show the reaction rate of pyrophyllite in sodium aluminate solution at different temperatures. Fig.8 shows that reaction rate increases with the raise of temperature while the reaction rate remains almost the same when the temperature is higher than 180 ℃. Fig.9 shows that the SiO2 concentration in solution increases gradually with the increase of time when the temperature is lower than 140 ℃. This indicates that pyrophyllite dissolves in this stage and serious scar is not formed. But, when the temperature is higher than 140 ℃, the SiO2 concentration reaches equilibrium quickly and begins to desilication. Especially when the temperature is higher than 170 ℃, the hydrate sodium aluminosilicate will precipitate soon and scar will be formed quickly.
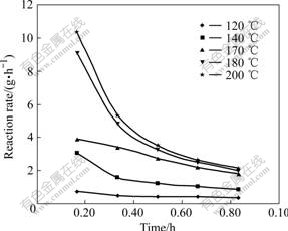
Fig.8 Change of reaction rate of pyrophyllite at different temperatures
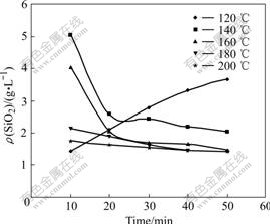
Fig.9 Effect of temperature on concentration of silicon dioxide in solution
5 Theoretical analysis on reaction properties of kaolinite, illite and pyrophyllite
According to the structure characteristics of kaolinite, illite, pyrophyllite and their complete cleavage plane, the electronic structure, charge number, atomic population and bond population were calculated by CASTEP (Cambridge Sequential Total Energy Package) program. The reaction behavior of kaolinite, illite and pyrophyllite in sodium aluminate solution can be determined by the analysis of those microscopic properties. CASTEP is a set of advanced quantum mechanics procedure developed by condensed matter theory research group of Cambridge University and can be used mainly in condensed matter system in the fields of chemistry and material science[16].
5.1 Calculation models and methods
Kaolinite, illite and pyrophyllite were selected as the research objects, and the calculation model was built according to the experimental lattice parameter. Fig.10 shows the calculation models of kaolinite, illite and pyrophyllite. Moreover, kaolinite, illite and pyrophyllite have complete cleavage planes which belong to (001) plane. Fig.11 shows the vacuum Slab calculation structure models for (001) plane of kaolinite, illite and pyrophyllite, respectively. The vacuum layer thickness of all Slab models was supposed to be 1.5 nm.
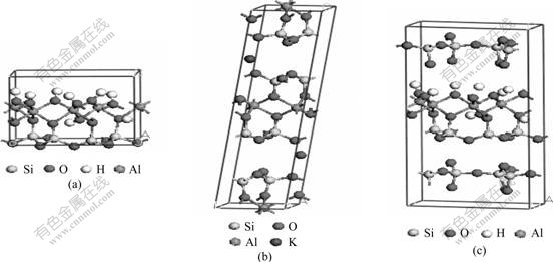
Fig.10 Calculated structure models of kaolinite (a), illite (b) and pyrophyllite (c)
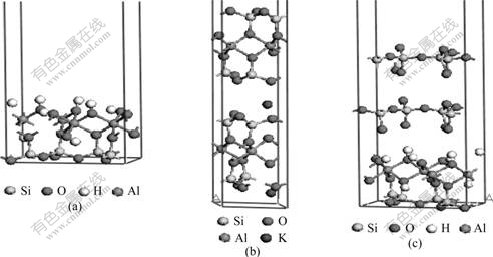
Fig.11 Calculated structure models of vacuum slab of kaolinite (a), illite (b) and pyrophyllite (c) at (001) plane
By using CASTEM procedure module, the structure models of kaolinite, illite, pyrophyllite and their ordinary complete cleavage planes were optimized at GGA-PW91 level. Ultrasoft pseudopotential (USP) was introduced to treat the interaction between electrons and ions. Pulay
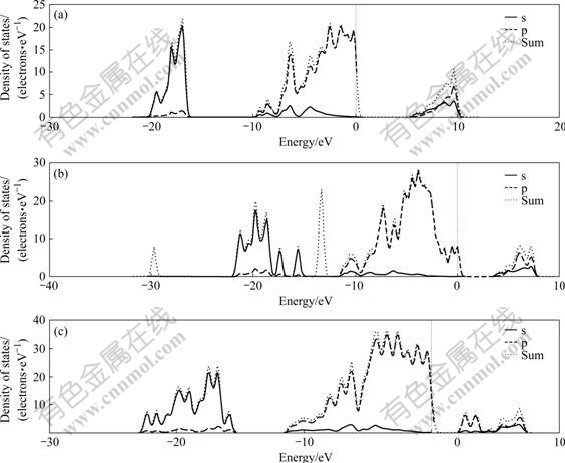
Fig.12 Densities of states of kaolinite (a), illite (b) and pyrophyllit (c)
Fig.13 shows that kaolinite, illite and pyrophyllite belong to sp belt and their PDOS are quite different, indicating the electronic structure difference of these three crystals. From the global aspect, the main component of minimum energy region group of kaolinite and pyrophyllite is s and a little p. Among the six energy regions of illite, the minimum energy region group contains onlys component and has only p component between the energy region from -14 to -13.5 eV. Table 1 shows that the average energy gap of pyrophyllite is the smallest, and that of the kaolinite is the largest, indicating that the reaction activity of pyrophyllite may be the greatest. The chemical property of kaolinite, illite and pyrophyllite can be inferred from Fig.13, such as the reaction activity may be quiet different, kaolinite is more stable and the reaction activity of pyrophyllite is relatively high.
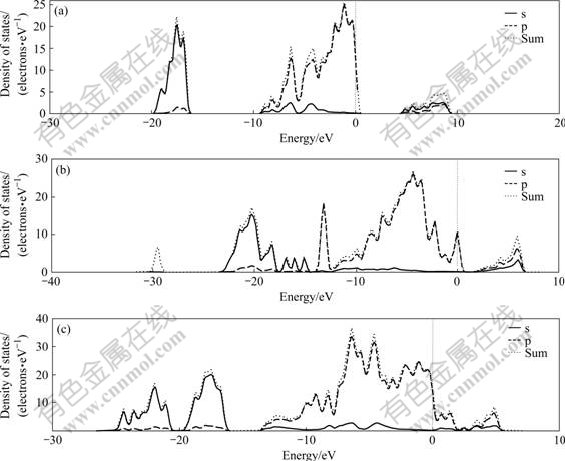
Fig.13 Densities of stages of slab of kaolinite (a), illite (b) and pyrophyllite (c) at 001 plane
Table 1 Energy and energy gap of k-points in first brillouin-zone for HOMO and LUMO of kaolinite, illite and pyrophyllite (Ha)

Fig.13 shows that the PDOS of (001) vacuum slab of kaolinite, illite and pyrophyllite are also quite different, which indicates that the electronic structure difference among the complete cleavage planes of the three crystals. The calculation results show that the surface activity of (001) plane of kaolinite, illite and pyrophyllite is higher than the inner. The surface activity of pyrophyllite is the largest among all of the research systems.
Actually, the removal ability of kaolinite in aluminate solution changes with its different occurrence states in bauxite. But generally all bauxite with kaolinite crystallizes quite well, so the pre-desiliconization effect under atmospheric pressure is poor and desiliconization ratio is only 25%-30%. Pure kaolinite crystallizes completely and has high crystallinity, so higher energy will be needed to break its crystal, that is pure kaolinite is relatively stable. According to our experiment results, the reaction activity of pyrophyllite in aluminate solution is apparently higher than that of illite, which is consistent with the calculation results.
5.3 Analysis of bond population of Si—O bond and Al—O bond in kaolinite, illite, pyrophyllite and their (001) plane slab
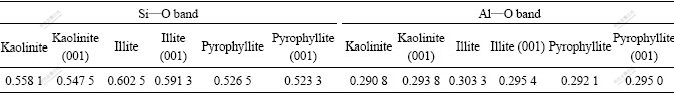
By using CASTEM procedure, the slab model of kaolinite, illite, pyrophyllite and their (001) plane were geometry optimized to obtain their bond population data of Si—O bond and Al—O bond.
According to the calculation results of Table 2, the intensity of surface Si—O bond of kaolinite, illite, pyrophyllite is slightly less than the internal Si—O bond, and the bonding force of surface Si—O bond is slightly less than the inner Si—O bond. Among kaolinite, illite and pyrophyllite, the average bond population of Si—O bond of illite is the largest and its bonding force of Si—O bond is also the largest. But the average bond population of Si—O bond of pyrophyllite is the smallest and its bonding force of Si—O bond is the smallest, so Si in pyrophyllite is more easy to be removed. The calculation results are consistent with our experiment results.
Table 2 Bond population of kaolinite, illite, pyrophyllite and their 001 planes
5.4 Theoretical calculation for reaction property of (001) plane of kaolinite, illite and pyrophyllite under alkaline condition
In order to analyze further the influence of alkaline on the chemical property of the (001) plane of kaolinite, illite and pyrophyllite, the surface action models between OH- and their (001) planes were set up (Fig.14) based on the theoretical calculation of the (001) plane slab.
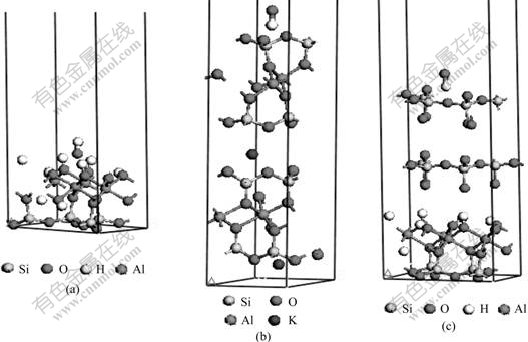
Fig.14 Calculated structure models of (001) slab of kaolinite (a), illite (b) and pyrophyllite (c) reacting with hydroxyl
The interaction between electron and ion was treated by USP. The energy calculation convergence precision was that the total energy was 2.0×10-5 eV/atom, and the plane wave cutoff energy was selected as 300.00 eV.
Fig.15 shows the PDOS calculation results of the action model among the (001) planes of kaolinite, illite, pyrophyllite and OH-. Table 3 shows the energy and energy gaps of every k-point of HOMO and LUMO of the action model among the (001) planes of kaolinite, illite, pyrophyllite and OH- in first Brillouin zone.
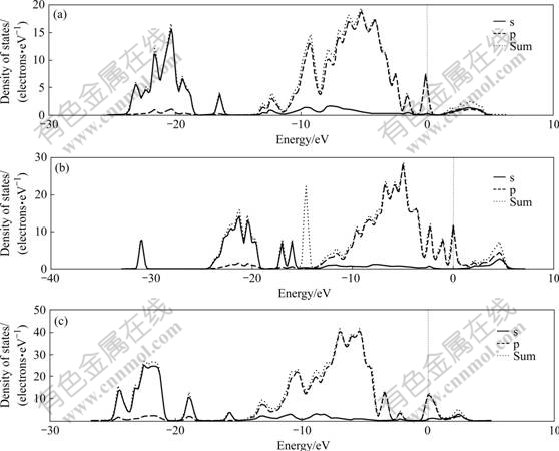
Fig.15 Densities of stages of (001) slab of kaolinite (a), illite (b) and pyrophyllite (c) reacting with hydroxyl
Table 3 HOMO and LUMO of (001) slab of kaolinite, illite and pyrophyllite reacting with hydroxyl (Ha)

The calculation results show that the existence of OH- has great influence on the electronic structure of the complete plane of kaolinite, illite and pyrophyllite, and OH- has the largest influence on the surface property of kaolinite.
The same analysis result can be obtained to compare the energy and energy gaps of every k-point of kaolinite, illite, pyrophyllite in first Brillouin zone with or without the existence of OH-.
Table 4 shows that the existence of OH- has great influence on the bonding force of Si—O bond of kaolinite, illite and pyrophyllite, and OH- has the largest influence on the Si—O bond of kaolinite.
Table 4 Band population and length of hydroxyl of kaolinite, illite, pyrophyllite 001 slabs
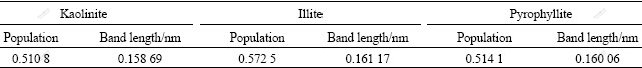
All of the above calculation results indicate and explain the experiment facts that OH- has great influence on kaolinite reactivity and also explain why kaolinite is more easy to be desiliconizated at relatively low temperature in alkaline solution even kaolinite has higher energy stability than pyrophyllite and illite. That is, the existence of OH- has the greatest influence on the surface property, electronic structure and Si—O bond population of kaolinite relatively.
6 Conclusions
1) Several single silicon-containing minerals including kaolinite, illite and pyrophyllite were systematically analysed and the digestion behaviors of these minerals in aluminate solution were investigated by simulating the industrial production condition. The results show that the digestion rates of kaolinite, illite, pyrophyllite in aluminate solution are influenced by reaction conditions such as temperature. The reaction rate of kaolinite in NaOH solution is much larger than that in aluminate solution under the same caustic alkali concentration.
2) By adopting quantum chemistry theoretical calculation and using CASTEM procedure module, the slab model of kaolinite, illite, pyrophyllite and their ordinary complete cleavage plane (001) were optimized at GGA-PW91 level. The difference of the reaction activities among kaolinite, illite, pyrophyllite in aluminate solution are opened out.
3) The microscopic electronic structure and energy bands character of kaolinite, illite and pyrophyllite are quite different, and the average energy gap of pyrophyllite is the smallest, indicating that the reaction activity of pyrophyllite is the highest. From the view of frontier orbital, the reaction activity of kaolinite, illite, pyrophyllite are obviously different and the reaction activity of pyrophyllite is the highest.
4) The calculation results of bond population show that the Si—O bond strength of illite is the strongest and its Al—O bond strength is comparatively the weakest, so Si in it is most difficult to be removed; The Si—O bond strength of pyrophyllite is the weakest, so Si in it is relatively more easy to be removed.
5) The electron structure of complete cleavage plane and the Si—O bond strength of kaolinite, illite and pyrophyllite are evidently influenced by the existence of OH-, of which the surface character and the reduction of Si—O bond strength of kaolinite are influenced greatly by OH-, therefore the desilication of kaolinite in alkaline solution at relatively lower temperature is easier.
References
[1] SWADDLE T W, ROSENQVIST J, YU P, BYLASKA E, PHILLIPS B L, CASEY W H. Kinetic evidence for five-coordination AlOH(aq)2+[J]. Science, 2005, 308(5727): 1450-1453.
[2] XIAO Ya-qing. Technology development of China’s aluminum industry[M]. Beijing: Metallurgical Industry Press, 2007: 37-38. (in Chinese)
[3] HUANG Guo-zhi, FANG Qi-xue, CUI Ji-rang. Beneficiation ways and research progress of bauxite ore[J]. Light Metals, 1999, 5: 16. (in Chinese)
[4] ZHAO Shi-min, WANG Dian-zuo, HU Yue-hua. Current study status of the pre-desilicif ication for bauxite[J]. Mining R&D, 2004, 10: 37-44. (in Chinese)
[5] BI Shi-wen, YU Hai-yan, YANG Yi-hong, ZHAO Fu-hui, YIN Zhong-lin. Production of alumina using Bayer process[M]. Beijing: Metallurgical Industry Press, 2007: 69-74. (in Chinese)
[6] BI Shi-wen, YU Hai-yan. Technology of alumina production[M]. Beijing: Chemical Industry Press, 2006: 65-69. (in Chinese)
[7] YIN Zhen-guo, ZHAO Qing-jie, CHEN Qi-yuan. Study of the desilication process of illite reacted with sodium aluminate solution [J]. Light Metals, 2005, 12: 40-44.
[8] YIN Zhong-Lin, BI Shi-wen, GU Song-qing. Study of reaction kinetics of Ti-containing minerals in preheating process ofbauxite slurry[J]. Mining and Metallurgical Engineering, 2005, 4: 23-27. (in Chinese)
[9] YIN Zhen-guo. Reaction behavior of several mono-silicon minerals in aluminate solution[R]. Changsha: Central South University, 2005: 25-29. (in Chinese)
[10] ROACH G I D, WHITE A J. Dissolution kinetics of kaolin in caustic liquors[C]// Warrendale: Light Metals, 1988: 41-47.
[11] LIU Gui-hua, HE Bo-quan, LI Xiao-bin, PENG Zhi-hong, ZHANG Chuang-fu. Study of reaction kinetics of kaolinite and sodium hydroxide[J]. Metallurgy of Nonferrous Metals, 1998(4): 30-32. (in Chinese)
[12] XU Qi-li. Effect of bauxite ores in different areas and impurity in solution on the behaviors of silicon and ferrous in bayer digestion process[C]// LIU Quan-pu. Symposium of 1st Academic Conference for Metallurgy of Light Metals. Zhengzhou: Academic Committee in Metallurgy of Light Metals, 1986: 126-137. (in Chinese)
[13] GU Song-qing. Reaction mechanism of illite in preheating bauxite slurry[C]// YIN Zhong-lin. Symposium of the 2nd Academic Conference of Metallurgy of Light Metals. Xining: Academic Committee of Metallurgy for Light Metals of Society of Chinese Nonferrous Metals, 1990: 128-135. (in Chinese)
[14] GU Song-qing, CAO Rong-jiang, CHEN Xin-min. Study on digestion kinetics of diasporic bauxite[J]. Acta of Metallurgica Sinica, 1987, 23(6): 269-274. (in Chinese)
[15] HU Yue-hua, WANG Min-hua. Floatation chemistry of aluminium silicate mineral and desilication of bauxite[M]. Beijing: Science and Technology Press, 2004: 21. (in Chinese)
[16] MILMAN V, WINKLER B, WHITE J A, PICKARD C J, PAYNE M C, AKHMATSKAYA E V, NOBES R H. Electronic structure, properties, and phase stability of inorganic crystals: a pseudopotential plane-wave study[J]. International Journal of Quantum Chemistry, 2000, 77(6): 895-910.
____________________________
Foundation item: Project(2005CB623702) supported by the National Basic Research Program of China
Corresponding author: ZHAO Qing-jie; Tel: +86-371-68918237; E-mail: zyy_zqj@rilm.com.cn
(Edited by YANG You-ping)


