Trans. Nonferrous Met. Soc. China 24(2014) 1452-1457
A novel wet-chemical method for preparation of silver flakes
Ai-xia ZHAI1, Xiong-hui CAI2, Bin DU3
1. School of Chemical and Environmental Engineering, Wuhan Polytechnic University, Wuhan 430023, China;
2. School of Biologic and Pharmaceutical Engineering, Wuhan Polytechnic University, Wuhan 430023, China;
3. China National Apparatus Research Institute, Guangzhou 510300, China
Received 27 May 2013; accepted 29 September 2013
Abstract:
A novel wet-chemical method for the preparation of silver flakes was studied. The well-defined particles were prepared by directly adding FeSO4 solution into AgNO3 solution containing citric acid at an agitation speed of 150 r/min at room temperature. The products were characterized by scanning electron microscopy (SEM) and X-ray diffraction (XRD). The results show that particles are irregular thin silver flakes. And the sizes of them range from 2 to 10 μm. It is found that citric acid plays an important role in the formation of sliver flakes. There is an optimum amount of citric acid for the preparation of silver flakes by this method. It is also found that high reduction rate is favorable for the formation of silver flakes.
Key words:
silver flake; wet-chemical method; FeSO4·7H2O; citric acid;
1 Introduction
The ultrafine metallic powders are used extensively in light, electricity, magnetism, heat and catalysis areas. And the silver powders had attracted considerable attention during the past few years for their many advantages, such as high electrical and thermal conductivity, high inoxidizability, excellent solderability and reasonable price. Among many preparation methods of silver particles, the wet-chemical method is often preferred for its advantage of the size and shape controlling. Nowadays, people have made many progresses in the wet-chemical synthesis of silver nanostructures with different shapes, such as nanocubes [1,2], nanodisks [3,4], nanoprisms [5-7], nanoplates [5,8-10], nanorods [11,12], and nanowires [1,13,14]. And people also succeeded in the synthesis of micro- sized silver particles with different shapes, such as micro-sized sphere [15,16] and micro-sized dendrites [17-19], by wet-chemical methods. They also developed some approaches for the preparation of micro-sized silver flake. DU et al [20] presented another thermal method to prepare silver sheets at 250 °C via decomposition of the silver nitrate in ethanol in the presence of ammonia. The lateral dimension of the silver sheets was up to 15 μm. And LIANG et al [21] synthesized silver flakes by reducing the tollen reagent with hydrogen peroxide under the catalysis of chloroplatinic acid in ethylene glycol. The size of the silver flakes could reach 30 μm. Obviously, the methods mentioned above for the preparation of micro-sized silver flakes need to be carried out in a thermal process or need seeds to catalyze and promote the anisotropic growth of silver nucleus. In our previous work [22], the micro-sized hexagonal and triangular silver plates were prepared by mixing of FeSO4·7H2O solution with silver nitrate solution in the presence of different modifiers at room temperature. The mean edge lengths of resulting products were 2 and 3 μm, respectively. But the product yield was very low. To promote the preparation of the silver flakes with the simple wet-chemical methods and enhance the understanding of the formation mechanism of silver crystals during these preparation processes, a simple approach to prepare the micro-sized silver flakes still needs to be demonstrated. In this work, a novel simple wet-chemical method for the preparation of micro-sized silver flakes is presented. It is carried out by directly adding the FeSO4·7H2O into silver nitrate solution at the presence of citric acid at room temperature.
2 Experimental
2.1 Materials
In this study, silver nitrate (AgNO3, analytical reagent, Shanghai Chemical Reagent Co., Ltd), Iron (II) sulfate heptahydrate (FeSO4·7H2O, analytical reagent, Sinopharm Chemical Regent Co., Ltd), citric acid (C6H8O7·H2O, analytical reagent, Shanghai Chemical Regent Co., Ltd), absolute ethanol (C2H5OH, analytical reagent, Shanghai Chemical Regent Co., Ltd) and de ionized water were used as the raw materials without further purification.
2.2 Method
In a typical process, 1 g AgNO3 and 4.4 g FeSO4·7H2O were dissolved in 50 mL de ionized water to prepare the solutions of them, respectively. And 0.15 g C6H8O7·H2O was added in the AgNO3 solution. Then the FeSO4·7H2O solution was added in AgNO3 solution immediately with an agitation speed of 150 r/min at room temperature. When the color of the mixed solution did not change, the reaction was ceased. After separated from the mixed solution, the precipitation was washed by de ionized water for 3-4 times and then by ethanol for 2-3 times. Then the powders were obtained after the precipitation was desiccated in the vacuum-desiccator at 50 °C for 30 min.
The powders were characterized by scanning electron microscopy (SEM, S-3000, Hitachi) and X-ray diffraction (XRD, X'Pert PRO, PANalytical B.V, Holland).
3 Results and discussion
3.1 Formation of silver flakes
The silver particles prepared in the typical process mentioned above were characterized by SEM and XRD. The SEM results are shown in Fig. 1. It shows that silver microcrystals mostly consist of irregular flakes (Fig. 1(a)). The flakes have smooth surfaces. And they are so thin that look like frangible. And the sizes of them range from 2 to 10 μm (Fig. 1(b)).
Figure 2 shows the XRD pattern of the particles. It reveals that the significant diffraction peaks locate at 38.3°, 44.4°, 64.6°, and 77.6°, which correspond to the characteristic peaks of (111), (200), (220), and (311) crystal planes of silver metal, respectively, indicating that the powders consist of pure crystalline silver.
The reduction of Ag+ by Fe2+ can be formulated as follows:
Ag++Fe2+=Ag+Fe3+ (1)
The formation process of silver microcrystals commonly includes the production of silver nucleus and the growth of silver crystals. And the growth process of silver crystals is often affected by the diffusion rates of components in the mixed solution [23]. It is found that the (111) plane of silver possesses the lowest surface energy, and it can absorb some suitable additives, such as citrate [4,9] and sulfate ion [24], which can further lower the surface energy and stabilize the silver plates with the (111) plane as the basal plane. Then the growth rate of silver crystals along the {111} facets is the fastest. As mentioned above, the growth rate is also affected by the diffusion rate of the silver atoms, which is decided by the reduction rate. According to Eq. (1), when the reduction rate is high, the new silver atoms will be produced instantly. It is easy for them to diffuse to the formed nucleus. The anisotropic growth of silver crystals will happen under the regulation of sulfate ion and citric acid. Then the silver flakes will be produced. If the reduction rate is low, the concentration of silver atoms will decrease. And the diffusion rate of silver atoms to the silver nucleus will decrease too, which will lead to the decrease of anisotropic growth rate of silver crystals. In this typical process, the mole ratio of FeSO4·7H2O to AgNO3 is much bigger than the theoretical value. It leads to a high reduction rate and a big concentration of silver atoms. Then under the regulation of sulfate ion and citric acid, the silver nucleus grow into flakes.
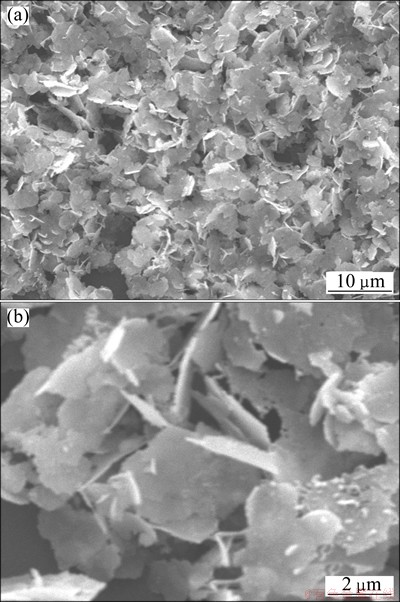
Fig. 1 SEM images of silver flakes with low magnification (a) and high magnification (b)
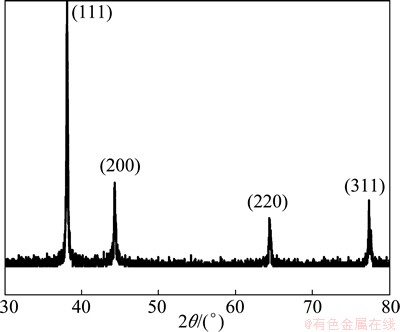
Fig. 2 XRD pattern of silver powders
3.2 Effect of amount of citric acid on formation of silver flakes
According to the mentioned formation mechanism, citric acid may be the key role in the formation of silver flakes. In order to investigate the effect of the amount of it on the morphologies of prepared silver powders, the silver powders were prepared at the cases of 0.07 and 0.3 g citric acid, respectively. And other conditions were identical to the description in section 2.2. The results are shown in Fig. 3.
It is clear that the shape of silver particles does not change much when the amount of citric acid decreases to 0.07 g from 0.15 g. The silver particles prepared at different conditions both consist of flakes. But the particle size decreases. It ranges from 1 to 5 μm when the amount of citric acid is 0.07 g. But when the amount of citric acid increases to 0.3 g, there are some flower-like silver crystals among the silver flakes. And the particle size decreases, too. The average size of them is about 3 μm. It can be concluded that citric acid plays an important role and there is an optimum value of it in the formation of big silver flakes.
In the formation of silver flakes, a low reduction rate will lead to a low concentration of silver atoms. And the growth of outshoots on the (111) planes or in other crystal facet directions is inevitable although the crystal growth rate along the {111} facets of silver nucleus is the fastest. The outshoots can act as new silver “nucleus” during the growth process of silver crystals. When the concentration of silver atoms is low, the diffusion rate of silver atoms to the silver nucleus, which grows into a silver flakes along the {111} facets, will decrease greatly. But at this time, the diffusion rate of silver atoms to the new silver nucleus can not be ignored for the shorter diffusion distances. And the silver atoms do not need to diffuse to the special spots to promote the anisotropic growth. Then newly arriving silver atoms can easily diffuse onto the surfaces of the new silver “nucleus”. Then they incorporate into these silver “nucleus” in an oriented direction that the (111) plane also serves as the basal plane under the regulation of citric acid and sulfate ion. Then secondary flakes with {111} facet terminations are formed and the hierarchical structures terminating with {111} facets are roughly developed. The mentioned growth processes are repeated. The third and the higher level flakes are also formed. Finally, the flower-like silver particles are produced. This formation process is similar to that of the silver and gold flower-like nano architectures with electrochemical approach [23,25].
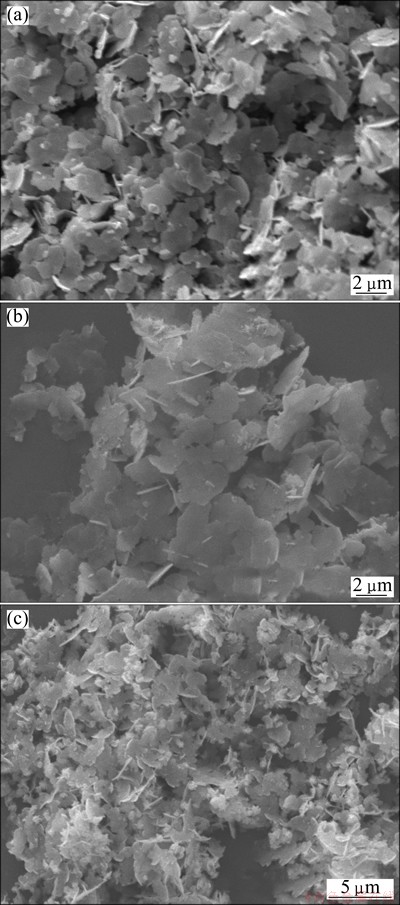
Fig. 3 SEM images of silver flakes obtained at citric acid of 0.07 g (a), 0.15 g (b) and 0.3 g (c)
When the amounts of citric acid are 0.07 and 0.15 g, the reduction rates are high for the low concentrations of citric acid. The complexation of citrate between Ag+ can happen, which can affect the reduction rate. The less citric acid is, the weaker the complexation is and the higher the reaction rate is. Then the effect of diffusion of silver atom can be ignored. Under the regulation of sulfate ions and citric acid, the silver nucleus grows into bigger silver flakes (Figs. 3(a) and (b)). And when the amount of citric acid decreases to 0.07, from 0.15 g, the complexation between the citrate and silver ions decreases. Then the reduction rate is higher, and more silver nucleus are produced at the initial reaction stage, which causes the size of silver flakes decreased (Fig. 3(a)). When the amount of citric acid increases to 0.3 g, the reduction rate decreases. And the concentration of silver atoms decreases. Then, the effect of the diffusion of silver atoms becomes apparent. It is beneficial for the formation of flower-like silver microcrystals (Fig. 3(c)).
3.3 Effect of concentration of FeSO4·7H2O solution on morphologies of silver particles
To clarify the behavior of the silver crystals growth at different concentrations of the reductant solution, systematic experiments were carried out at different concentrations of FeSO4·7H2O solution. The silver particles were synthesized at the concentrations of FeSO4·7H2O solution of 2.0 and 1.0 g per 50 mL deionized water, respectively. And other conditions are the same as those described in Section 2.2. The results are shown in Fig. 4.
It is obvious that the concentration of FeSO4·7H2O solution has a huge impact on the morphologies of silver powders. With the decreasing of the concentration of FeSO4, the amount of silver flakes and the flake size both decrease. And there are more flower-like microcrystals. When the concentration of FeSO4·7H2O solution decreases (Figs. 4(b) and (c)), the reduction rate decreases. And the mediation of sulfate ions on crystal growth also decreases. It is beneficial for the formation of the flower-like silver microcrystals. And the more the concentration of FeSO4·7H2O solution decreases, the more the proportion of flower-like silver microcrystals is. It is similar to the results caused by the increase of amount of citric acid from 0.15 to 0.3 g. And it implies that it is favorable to obtain big silver flakes at high concentration of FeSO4·7H2O solution by this proposed method.
3.4 Effect of adding method of FeSO4·7H2O solution on morphologies of silver particles
In order to investigate the effect of the adding method of FeSO4·7H2O solution into AgNO3 solution on the morphologies of silver powders, two kinds of particles were prepared when FeSO4·7H2O solutions were poured in and dripped in AgNO3 solution, respectively. That is, one kind of powder was prepared according to the method described in Section 2.2, and the other was prepared when the FeSO4·7H2O solution was added into the AgNO3 solution drop by drop in 20 min. The other conditions were identical. The results are shown in Fig. 5.
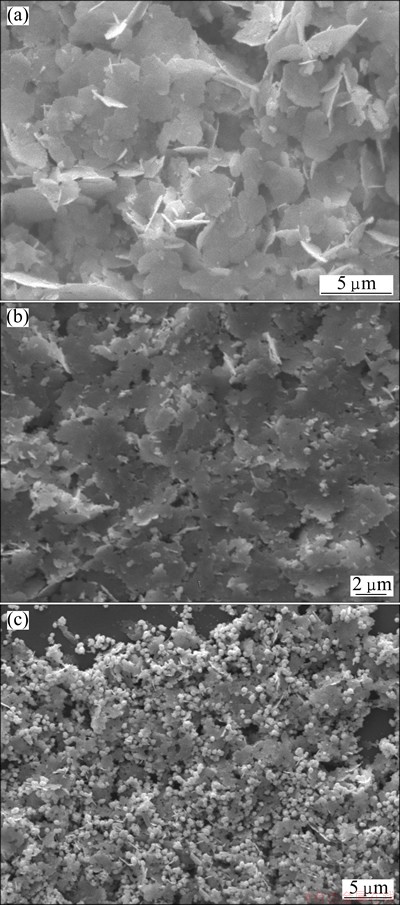
Fig. 4 SEM images of silver flakes obtained at FeSO4·7H2O solution of 4.4 g (a), 2.0 g (b) and 1.0 g (c)
It is clear that the morphologies of microcrystals change greatly. When the FeSO4·7H2O solution is dripped in AgNO3 solution, the powders consist of flower-like silver crystals. And there are a few silver flakes. The diameter of flower-like silver crystals is about 1 μm. And many of them agglomerate together. When the FeSO4·7H2O solution is added into the AgNO3 solution by dripping, the concentration of FeSO4·7H2O is lower than that by pouring method. So the reduction rate decreases and the concentration of silver atoms also decreases. And similar to the description in Section 3.3, the crystal-growth mediation of sulfate ion becomes weaker for its low concentration. So it is favorable for the formation of silver flower-like microcrystals. And it is similar to the case that the concentration of FeSO4·7H2O solution is 1.0 g.
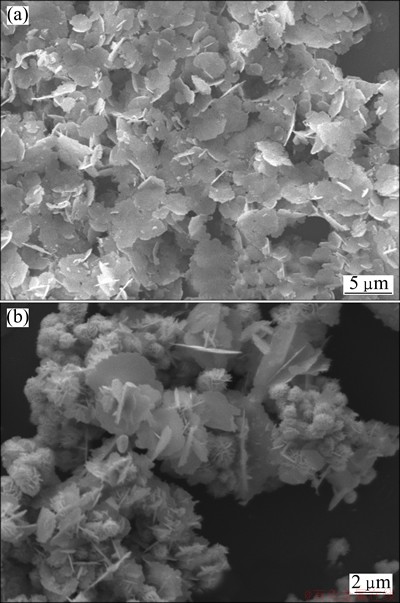
Fig. 5 SEM images of silver microcrystals obtained with FeSO4·7H2O solution by pouring in (a) and dripping in (b) AgNO3 solution
4 Conclusions
1) The silver flakes were prepared by directly adding FeSO4·7H2O solution into AgNO3 solution containing citric acid at ambient temperature.
2) It was found that the citric acid promoted the anisotropic growth of the silver nucleus into flakes through its capping and cooperation mediation with sulfate ion on silver nucleus growth and complexation between Ag+. There was an optimum value of it in the formation of big silver flakes.
3) The flower-like silver particle could be obtained when the reduction rate was enough low in this proposed method, which was caused by the low diffusion rate of silver atoms during the growth of silver crystals.
References
[1] ZHANG W C, WU X L, CHEN H T, GAO Y J, ZHU J, HUANG G S, CHU P K. Self-organized formation of silver nanowires, nanocubes and bipyramids via a solvothermal method [J]. Acta Mater, 2008, 56(11): 2508-2513.
[2] ZHAO Tian, FAN Jun-bing, CUI Jing, LIU Jin-hua, XU Xiao-bo, ZHU Ming-qian. Microwave-controlled ultrafast synthesis of uniform silver nanocubes and nanowires [J]. Chem Phys Lett, 2011, 501(4-6): 414-418.
[3] TANG Bin, AN Jing, ZHENG Xian-liang, XU Shu-ping, LI Dong-mei, ZHOU Ji, ZHAO Bing, XU Wei-qing. Silver nanodisks with tunable size by heat aging [J]. J Phys Chem C, 2008, 112(47): 18361-18367.
[4] MAILLARD M, HUANG P, BRUS L. Silver nanodisk growth by surface plasmon enhanced photoreduction of adsorbed [Ag+] [J]. Nano Lett, 2003, 3(11): 1611-1615.
[5] DARMANIN T, NATIVO P, GILLILAND D, CECCONE G, PASCUAL C, BERARDIS B D, GUITTARD F, ROSSI F. Microwave-assisted synthesis of silver nanoprisms/nanoplates using a “modified polyol process” [J]. Colloid Surf A-Physicochem Eng Asp, 2012, 395: 145-151.
[6] TANG Bin, XU Shu-ping, AN Jing, ZHAO Bing, XU Wei-qing. Photoinduced shape conversion and reconstruction of silver nanoprisms [J]. J Phys Chem C, 2009, 113(17): 7025-7030.
[7] CATHCART N, KITAEV V. Monodisperse hexagonal silver nanoprisms: Synthesis via thiolate-protected cluster recursors and chiral, ligand-imprinted self-assembly [J]. ACS Nano, 2011, 5(9): 7411-7425.
[8] ROH J, YI J, KIM Y. Rapid, Reversible preparation of size-controllable silver nanoplates by chemical redox [J]. Langmuir, 2010, 26(14): 11621-11623.
[9] ZHANG Qiao, LI Na, GOEBL James, LU Zhen-da, YIN Ya-dong. A systematic study of the synthesis of silver nanoplates: Is citrate a “magic” reagent? [J]. J Am Chem Soc, 2011, 133(46): 18931-18939.
[10] HUANG L , ZHAI Y, DONG S, WANG J. Efficient preparation of silver nanoplates assisted by non-polar solvents [J]. J Colloid Inter Sci, 2009, 331(2): 384-388.
[11] NADAGOUDA M N, VARMA R S. Microwave-assisted shape-controlled bulk synthesis of Ag and Fe nanorods in poly (ethylene glycol) solutions [J]. Cryst Growth Des, 2008, 8(1): 291-295.
[12] ZHANG J, LANGILLE M R, MIRKIN C A. Synthesis of silver nanorods by low energy excitation of spherical plasmonic seeds [J]. Nano Lett, 2011, 11(6): 2495-2498.
[13] YANG Zhi-qiang, QIAN Hai-jun, CHEN Hong-yu, ANKER J N. One-pot hydrothermal synthesis of silver nanowires via citrate reduction [J]. J Colloid Inter Sci, 2010, 352(2): 285-291.
[14] ZHAI Ai-xia, CAI Xiong-hui, JIANG Xiao-ye, FAN Guo-zhi. A novel and facile wet-chemical method for synthesis of silver microwires [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(4): 943-948.
[15] AHN J G, KIM D J, LEE J R, JUNG H S, KIM B G. Synthesis of mono-dispersed fine spherical silver powders by chemical reduction method [J]. Mater Sci Forum, 2007, 539-543(3): 2782-2786.
[16] AN Bing, CAI Xiong-hui, WU Feng-shun, WU Yi-ping. Preparation of micro-sized uniform spherical Ag powders by novel wet-chemical method [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(8): 1550-1554.
[17] QIU T, WU X L, WAN G J, MEI Y F, SIU G G, CHU P K. Self-organized synthesis of micrometer scale silver disks by electroless metal deposition on Si-incorporated diamond-like carbon films [J]. J Cryst Growth, 2005, 284(3-4): 470-476.
[18] BHATTACHARYA S, DAS A K, BANERJEE A, CHAKRAVORTY D. Dendron-like growth of silver nanoparticles using a water-soluble oligopeptide [J]. J Phys Chem B, 2006, 110(22): 10757-10761.
[19] FANG Ji-xiang, YOU Hong-jun, KONG Peng, YI Yan, SONG Xiao-ping, DING Bing-jun. Dendritic silver nanostructure growth and evolution in replacement reaction [J]. Cryst Growth Des, 2007, 7(5): 864-867.
[20] DU Ji-min, HAN Bu-xing, LIU Zhi-min, LIU Yun-qi. Control synthesis of silver nanosheets, chainlike sheets, and microwires via a simple solvent-thermal method [J]. Cryst Growth Des, 2007, 7(5): 900-904.
[21] LIANG H, KIM D, CHUNG H, ZHANG J, YU K, LI S, LI R. Mechanism for the formation of flake silver powder synthesized by chemical reduction in ethylene glycol [J]. Acta Phys Chim Sin, 2003, 19(2): 150-153.
[22] CAI Xiong-hui, ZHAI Ai-xia. Preparation of micro-sized silver crystals with different morphologies by a wet-chemical method [J]. Rare Metals, 2010, 29(4): 407-412.
[23] TANG Shao-chun, MENG Xiang-kang, WANG Chang-chun, CAO Zhen-hua. Flowerlike Ag microparticles with novel nanostructure synthesized by an electrochemical approach [J]. Mater Chem Phys, 2009, 114(1-2): 842-847.
[24] PATRITO M, PAREDES O, SELLERS H. On the nature of the 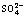 /Ag (111) and
/Ag (111) and 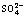 /Au (111) surface bonding [J]. Surf Sci,1997, 380(2-3): 264-282.
/Au (111) surface bonding [J]. Surf Sci,1997, 380(2-3): 264-282.
[25] DUAN Guo-tao, CAI Wei-ping, LUO Yuan-yuan, LI Zhi-gang, LI Yue. Electrochemically induced flowerlike gold nanoarchitectures and their srong surface-enhanced Raman scattering effect [J]. Appl Phys Lett, 2006, 89(21): 2211905-1-3.
一种新颖的制备片状银粉的湿化学法
翟爱霞1,蔡雄辉2,杜 斌3
1. 武汉轻工大学 化学与环境工程学院,武汉 430023;
2. 武汉轻工大学 生物与制药工程学院,武汉 430023;
3. 中国电器科学研究院,广州 510300
摘 要:提出一种片状银粉的湿化学制备方法。在室温下,搅拌速度为150 r/min时,把FeSO4·7H2O溶液倒入含有柠檬酸的AgNO3溶液中制备银微晶体。采用扫描电镜(SEM)和X射线衍射仪(XRD)对制备的银微晶体进行表征。结果表明:制备的银微晶体主要由大量的不规则形状的片状银粉组成,其尺寸为2~10 mm;柠檬酸在片状银粉的形成过程中起关键作用,其用量对片状银粉的形成存在一个最佳值;当体系的还原速率较大时,更有利于片状银粉的形成。
关键词:片状银粉;湿化学法;硫酸亚铁;柠檬酸
(Edited by Chao WANG)
Foundation item: Project (B20121806) supported by the Science and Technology Research Program of Education Department of Hubei Province, China
Corresponding author: Ai-xia ZHAI; Tel: +86-86-27-83943956; E-mail: zaisfte@163.com
DOI: 10.1016/S1003-6326(14)63212-X
Abstract: A novel wet-chemical method for the preparation of silver flakes was studied. The well-defined particles were prepared by directly adding FeSO4 solution into AgNO3 solution containing citric acid at an agitation speed of 150 r/min at room temperature. The products were characterized by scanning electron microscopy (SEM) and X-ray diffraction (XRD). The results show that particles are irregular thin silver flakes. And the sizes of them range from 2 to 10 μm. It is found that citric acid plays an important role in the formation of sliver flakes. There is an optimum amount of citric acid for the preparation of silver flakes by this method. It is also found that high reduction rate is favorable for the formation of silver flakes.


