Trans. Nonferrous Met. Soc. China 23(2013) 3324-3329
Improving electrochemical performances of LiFePO4/C cathode material via a novel three-layer electrode
Zheng-wei XIAO1, Guo-rong HU2, Ke DU2, Zhong-dong PENG2
1. Faculty of Metallurgical and Energy Engineering, Kunming University of Science and Technology, Kunming 650093, China;
2. School of Metallurgy and Environment, Central South University, Changsha 410083, China
Received 22 October 2012; accepted 10 April 2013
Abstract:
As an improvement on the conventional two-layer electrode (active material layer|current collector), a novel sandwich-like three-layer electrode (conductive layer|active material layer|current collector) for cathode material LiFePO4/C was introduced in order to improve its electrochemical performance. LiFePO4/C in the three-layer electrode exhibited superior rate capability in comparison with that in the two-layer electrode in accordance with charge-discharge examination. Cyclic voltammetry and electrochemical impedance spectroscopy indicated that Fe3+/Fe2+ redox couple for LiFePO4 in the three-layer electrode displayed faster kinetics, better reversibility and much lower charge transfer resistance than that in the two-layer electrode in electrochemical process. For three-layer electrode, the holes in the surface of active material layer were filled by smaller acetylene black grains, which formed electrical connections and provided more pathways to electron transport to/from LiFePO4/C particles exposed to the bulk electrolyte.
Key words:
lithium ion cells; LiFePO4; cathode electrode configuration; three-layer electrode; electrochemical performances;
1 Introduction
Owing to the advantages of cheap raw materials, environmental friendliness, good cycle stability and appreciable capacity of 170 mA·h/g, olivine LiFePO4 has been qualified as one of the most promising cathode materials for lithium ion cells of next generation [1-4]. But compared with the electronic conductivities of LiCoO2 and LiMn2O4, 10-3 S/cm and 2×10-5-5×10-5 S/cm respectively, the corresponding value of LiFePO4 is 10-9-10-10 S/cm [5]. Therefore, pristine LiFePO4 is electroactive only at a very low current density, and its direct application is hampered. So far, the techniques applied to improve the poor rate capability of pristine LiFePO4 have mainly focused on nano-sizing [6], conductive coating [7,8] and ion doping [9] to treat LiFePO4 itself. All the ameliorations can enhance the rate performance of LiFePO4. According to our laboratorial experience, the synthesis of LiFePO4/C is simple to realize.
A lot of work has been carried out to optimize the experimental conditions for LiFePO4/C preparation and carbon content, while few research groups have tried to alter the following fabrication for cathode electrode. As a matter of fact, the improvement on the mass ratio for LiFePO4/C, PVdF binder and electrical conductor [10], and the use of porous current collector [11] can further improve the electrochemical performance of LiFePO4, which indicates electrode configuration for LiFePO4 should be an indispensable part of systematic research on LiFePO4 for its application.
In this work, as an improvement on the conventional two-layer configuration, an additional layer of acetylene black film on the surface of active material layer was applied to derive a novel sandwich-like three-layer electrode. The electrochemical performance of LiFePO4/C cathode material can be substantially elevated in the three-layer electrode in comparison with that in the conventional two-layer electrode. The appreciable capacities for LiFePO4/C in the three-layer electrode at high rates are comparable to the reported [12], even though optimization for its preparation has not been made.
2 Experimental
Li2CO3, FeC2O4·2H2O and NH4H2PO4 with molar ratio of 1:2:2 and a certain amount of glucose were ball-milled in anhydrous ethanol for 2.5 h with a speed of 300 r/min. The slurry obtained was dried, and the dried lump was sintered at 650 °C in an argon atmosphere for 24 h. After furnace cooling to room temperature, LiFePO4/C was obtained.
The carbon content for LiFePO4/C was detected according to Ref. [13]. The X-ray powder diffraction (XRD) pattern for LiFePO4/C was derived on Philips X-pert powder diffractometer using Cu Kα radiation. Scanning electron microscopy (SEM, JEOL JSM- 5600LV) was used to observe the surface of the two-layer electrode and the surface and cross-section of the three-layer electrode based on the same LiFePO4/C.
LiFePO4/C, PVdF binder and acetylene black with mass ratio of 8:1:1 were ground with the addition of NMP solvent. The slurry was then uniformly spread over an aluminum foil current collector, which was dried at 120 °C under vacuum. For the three-layer electrode, a slurry containing acetylene black and PVdF with mass ratio of 2:1 obtained using NMP solvent was evenly coated onto the dried two-layer electrode, which was also dried at 120 °C under vacuum. Coin cells 2025 using three-layer or two-layer cathode electrodes were assembled in an argon-filled glovebox. The electrolyte was 1 mol/L LiPF6 in a mixture of EC, EMC and DEC with volume ratio of 1:1:1. Metallic lithium was used as the counter electrode, and Celgard 2400 porous membrane was used as separator. At room temperature, LiFePO4/C in three-layer or two-layer electrodes was galvanostatically examined at different rates in the voltage window of 2.5-4.1 V on Land BTI-40. Specific capacity calculation was based on the mass of LiFePO4/C loaded in the cathode.
Cyclic voltammetry (CV) was performed with a Potentiostat/Galvanostat Model 273A. Three-electrode system was measured by CV measurements. The working electrode was three-layer or two-layer electrode which had undergone 0.25C charge-discharge process ten times, and metallic lithium was the counter and reference electrodes. Electrochemical impedance spectroscopy (EIS) for fully discharged three-layer or two-layer electrodes was carried out on Potentiostat/ Galvanostat Model 273A with Model 5210 lock-in amplifier.
3 Results and discussion
3.1 Structure and carbon content of LiFePO4/C
Figure 1 shows the XRD pattern for the as-prepared LiFePO4/C (6.4% (mass fraction) carbon). The curve is indexed in the orthorhombic space group Pnma. The peaks on the pattern are sharp and perfect, suggesting a high degree of crystallinity for the as-prepared LiFePO4. The pattern agrees very well with that of triphylite (JCPDS No. 40-1499), and no impurity phases can be detected.
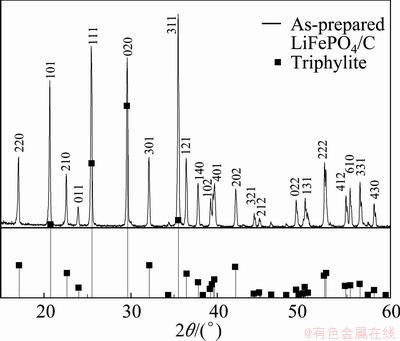
Fig. 1 XRD patterns for as-prepared LiFePO4/C and triphylite
3.2 SEM observation of two-layer and three-layer electrodes
The SEM images for the surface of the two-layer electrode, and the surface and cross-section of the three-layer electrode are shown in Fig. 2. Figures 2(a1) and (a2) show a coarse surface littered with LiFePO4/C particles between which there are holes, resulting from the evaporation of NMP solvent and precipitation of PVdF binder in electrode drying. Smaller acetylene black grains in deep black color can be observed to evenly distribute in electrode in Fig. 2(a1). The three-layer electrode is plain with just few pores far smaller in size than those in the surface of two-layer electrode, as shown in Figs. 2(b1) and (b2). An electrode with a planar surface can evenly distribute current and exhibits lower polarization than one with a coarse surface in electrochemical process. As shown in Fig. 2(c2), the LiFePO4/C particles in the surface of active material layer are well in contact with acetylene black grains filling up the superficial holes. Except for the surface, no big holes in the active material layer are observed, indicating the even distribution of LiFePO4/C particles and acetylene black grains in the active material layer, as shown in Fig. 2(c1).
3.3 Rate performances of LiFePO4/C in two-layer and three-layer electrodes
In Fig. 3(a), the typical charge-discharge profiles for LiFePO4/C in the three-layer and two-layer electrodes at 0.25C and 1C are indicated. The 0.25C charge-discharge curves for both electrodes show a flat plateau at around 3.45 V (vs Li+/Li), which is the voltage at which both the lithium insertion and extraction at room temperature proceed in a two-phase reaction between LiFePO4 and FePO4 [14]. At 0.25C, the discharge capacities for LiFePO4/C in the three-layer and two-layer electrodes are 151.2 and 149.8 mA·h/g, respectively, showing a negligible difference of 1.4 mA·h/g, but at 1C, the corresponding values are 143.5 and 125.7 mA·h/g, respectively, demonstrating a bigger difference of 17.8 mA·h/g, a value comprising more than 10% of the theoretical capacity of 170 mA·h/g.
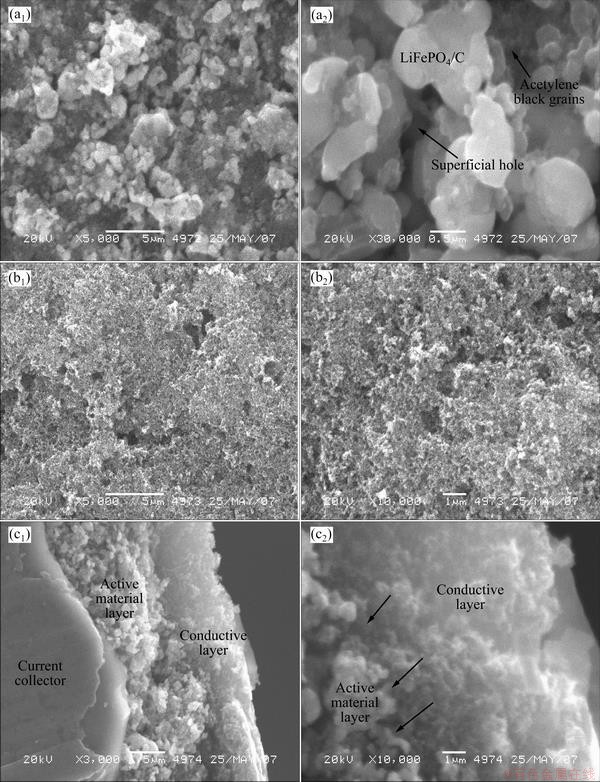
Fig. 2 SEM images of surface of two-layer cathode electrode (a1) and (a2), surface (b1, b2), cross section (c1, c2) of three-layer cathode electrode with arrows in (c2) indicating holes in the surface of active material layer being filled up by acetylene black grains
It is worth noting that at 0.25C the discharge curve for the three-layer electrode exhibits a slightly higher voltage plateau than that for the two-layer one, but at 1C the difference between the two electrodes’ discharge plateaus is significant. At 1C, the discharge voltage for the two-layer electrode is sloping down much faster than that for the three-layer electrode. In addition, the charge-discharge plateau voltages for the three-layer electrode are much better kept at around 3.45 V than those for the two-layer electrode. The results above suggest that three-layer electrode configuration can reduce the polarization during the charge-discharge process, in which the higher the rate is, the larger the polarization is. Therefore, the advantageous effect of the improved electrode configuration on the charge- discharge performance of LiFePO4/C is reflected in longer charge-discharge plateaus and well-kept charge- discharge plateau voltage at around 3.45 V, which are indicative of improved electrochemical performance.
Further evidence for the positive effect of three-layer configuration on the electrochemical performance of LiFePO4/C cathode material is shown in Fig. 3(b). From low rate 0.25C to high rate 2C, the discharge capacities for LiFePO4/C in both electrodes drop more or less, respectively, but in comparison, LiFePO4/C in the three-layer electrode exhibits a much higher discharge capacity than that in the two-layer electrode at the same rate. Even at 3C, LiFePO4/C in the three-layer electrode displays a discharge capacity of 115 mA·h/g, which is comparable to that reported in a novel work [12]. Meanwhile, at 2.5C and 3C, the two-layer electrode can be hardly discharged in the voltage window of 2.5-4.1 V, which is due to the immense polarization exhibited in a high current density process. The work shows that betterment in electrode configuration can be supplementary to modification on pristine LiFePO4 to further improve its rate capability.

Fig. 3 Charge-discharge profiles (a) and rate capacities (b) for LiFePO4/C cathode material in three-layer and two-layer electrodes
3.4 CV and EIS measurements on two-layer and three-layer electrodes
There is a significant difference between the CV curves for the two electrodes based on the same LiFePO4/C sample but with different configurations as shown in Fig. 4. For the Fe3+/Fe2+ redox couple in the three-layer electrode, the redox peaks are located at 3.63 and 3.24 V, respectively, with a difference of 0.39 V, and the corresponding values for that in two-layer electrode are 3.78 and 3.06 V, respectively, with a much bigger difference of 0.72 V, indicating a much smaller polarization for three-layer electrode than two-layer electrode at the same current density.

Fig. 4 Cyclic voltammegrams for three-layer and two-layer electrodes based on same LiFePO4/C sample at scanning rate of 0.1 mV/s
In addition, the redox peaks for Fe3+/Fe2+ redox couple in the three-layer electrode are sharper and higher than those in the two-layer electrode. All above indicate that Fe3+/Fe2+ redox couple in the three-layer electrode exhibits faster kinetics and better reversibility in electrochemical process. Therefore, three-layer electrode configuration facilitates redox, reduces polarization and increases reversibility for Fe3+/Fe2+ redox couple in electrochemical process.
The typical Nyquist and Bode plots for the two electrodes are shown in Fig. 5. The depressed semicircle in the moderate frequency region is attributable to the charge transfer process, and the tilted straight line in low frequency region corresponds to the Li+ diffusion process. The numerical value of the diameter of the semicircle on the Zreal axis is approximately equal to the charge transfer resistance (Rct) or the so-called interfacial resistance [15].
Figure 5(a) shows a significant drop in the interface impedance, from 1500 to 250 Ω, observed after the addition of the conductive layer. In the Bode plot of Fig. 5(b), the three-layer electrode also exhibits much smaller impedance than the two-layer electrode in the frequency range of 0.01-1000 Hz. Thus, the improvement in the electrode configuration facilitates charge transfer in electrochemical process for LiFePO4/C cathode material and hence reduces electrode polarization.

Fig. 5 Nyquist (a) and Bode (b) plots for three-layer and two-layer electrodes based on same LiFePO4/C sample
3.5 Improved electrochemical performances of LiFePO4/C due to three-layer configuration
The higher rate capacity, faster kinetics, better reversibility and lower charge transfer resistance for the three-layer electrode than those for the two-layer electrode can be attributed to the improved electrode configuration. For Li+ extraction from and insertion into LiFePO4 crystals in electrode process, equivalent number of electrons are involved at the same spot. By contrast, if LiFePO4 particles are not in good contact with electrical conductor, the transport of electrons to/from these spots will be reflected in additional polarization and, hence, in a lower reversible capacity [16].
Generally, none of the routes employed to synthesize LiFePO4/C can achieve a perfectly continuous carbon coating on each one of the LiFePO4 particles. As shown in Fig. 6(a), if the LiFePO4 particles exposed to the bulk electrolyte are not well-coated with carbon, the transport of electrons to/from LiFePO4 crystals in them will be exclusively dependent on LiFePO4/C particles themselves. Therefore, it will be a reason for sluggish electronic kinetics for those LiFePO4 crystals in electrochemical process. To the LiFePO4/C particles inner the active material layer, their contacts with evenly distributed acetylene black grains can counterbalance the adversity of unavoidable carbon discontinuity. In Fig. 6(b), conductive layer ensures electron transport to/from these superficial LiFePO4/C particles through more pathways, which apparently leads to faster electron transport to/from those LiFePO4 crystals in them. So LiFePO4/C in the three-layer electrode substantially exhibits improved electrochemical performances mainly because of the faster electronic kinetics.

Fig. 6 Sketch for two-layer (a) and three-layer (b) cathode electrodes with arrows indicating pathways for electron transport during charge-discharge process
Because of the promising LiFePO4 cathode material, the conventional two-layer electrode configuration is inadequate which is only suitable for those (LiCoO2 and LiMn2O4) with sufficiently high conductivities. The three-layer electrode concept is absolutely different from just improving acetylene black content in the two-layer electrode, for the conductive layer can be reduced to be as thin as it can be, which will lead to very limited influence on the density of the cathode. With no doubt, the improvement in electrode configuration is a very important part of the work for the commercialization of LiFePO4 as a cathode material for lithium ion cells.
4 Conclusions
1) Distinct from the reported techniques to modify pristine LiFePO4, a sandwich-like three-layer electrode (conductive layer|active material layer|current collector), for the cathode material has been introduced to improve its electrochemical performance.
2) LiFePO4/C in the three-layer electrode shows a much better rate capability than that in the two-layer electrode according to charge-discharge examination. In three-layer electrode, Fe3+/Fe2+ redox couple in the cathode material exhibits faster kinetics, better reversibility and lower charge transfer resistance than that in the two-layer electrode in accordance with CV and EIS measurements.
3) In three-layer electrode, the conductive layer forms conductive connections between superficial LiFePO4/C particles in active material layer and provides more pathways for electron transport to/from LiFePO4 crystals in them in electrochemical process. As a result, electrons can be faster transported to/from those active material crystals.
References
[1] LI Y D, ZHAO S X, NAN C W, LI B H. Electrochemical performance of SiO2-coated LiFePO4 cathode materials for lithium ion battery [J]. Journal of Alloys and Compounds, 2011, 509(3): 957-960.
[2] XIAO Zheng-wei, HU Guo-rong, DU Ke, PENG Zhong-dong, DENG Xin-rong. High density LiFePO4/C composite cathode material for lithium ion batteries [J]. The Chinese Journal of Nonferrous Metals, 2007, 17(12): 2040-2045. (in Chinese)
[3] KIM J, KIM H, PARK I, PARK Y U, YOO J K, PARK K Y. LiFePO4 with an alluaudite crystal structure for lithium ion batteries [J]. Energy and Environmental Science, 2013, 6(3): 830-834.
[4] ZHOU X, WANG F, ZHU Y, LIU Z. Graphene modified LiFePO4 cathode materials for high power lithium ion batteries [J]. Journal of Materials Chemistry, 2011, 21(10): 3353-3358.
[5] CHUNG S Y, BLOKING J T, CHIANG Y M. Electronically conductive phospho-olivines as lithium storage electrodes [J]. Nature Materials, 2002, 1(2): 123-128.
[6] LIU Y H, ZHANG W, ZHU Y J, LUO Y T, XU Y H, BROWN A, CULVER J N, LUNDGREN C A, XU K, WANG Y, WANG C S. Architecturing hierarchical function layers on self-assembled viral templates as 3D nano-array electrodes for integrated Li-ion microbatteries [J]. Nano Letters, 2013, 13(1): 293-300.
[7] SU L W, JING Y, ZHOU Z. Li ion battery materials with core-shell nanostructures [J]. Nanoscale, 2011, 3(10): 3967-3983.
[8] TANG Hao, TAN Long, XU Jun. Synthesis and characterization of LiFePO4 coating with aluminum doped zinc oxide [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(2): 451-455.
[9] DING Yan-huai, ZHANG Ping. Effect of Mg and Co-doping on electrochemical properties of LiFePO4 [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(10): s153-s156.
[10] ZANE D, CAREWSKA M, SCACCIA S, CARDELLINI F, PROSINI P P. Factor affecting rate performance of undoped LiFePO4 [J]. Electrochimica Acta, 2004, 49(25): 4259-4271.
[11] YAO M, OKUNO K, IWAKI T, KATO M, TANASE S, EMURA K, SAKAI T. LiFePO4-based electrode using micro-porous current collector for high power lithium ion battery [J]. Journal of Power Sources, 2007, 173(1): 545-549.
[12] XU Z H, XU L, LAI Q Y, JI X Y. Microemulsion synthesis of LiFePO4/C and its electrochemical properties as cathode materials for lithium-ion cells [J]. Materials Chemistry Physics, 2007, 105(1): 80-85.
[13] BELHAROUAK I, JOHNSON C, AMINE K. Synthesis and electrochemical analysis of vapor-deposited carbon-coated LiFePO4 [J]. Electrochemistry Communications, 2005, 7(10): 983-988.
[14] HU Guo-rong, XIAO Zheng-wei, PENG Zhong-dong, DU Ke, DENG Xin-rong. Preparation of LiFePO4 for lithium ion battery using Fe2P2O7 as precursor [J]. Journal of Central South University of Technology, 2008, 15(4): 531-534.
[15] LIU H, CAO Q, FU L J, LI C, WU Y P, WU H Q. Doping effects of zinc on LiFePO4 cathode material for lithium ion batteries [J]. Electrochemistry Communications, 2006, 8(10): 1553-1557.
[16] DOMINKO R, GABERSCEK M, DROFENIK J, BELE M, PEJOVNIK S, JAMNIK J. The role of carbon black distribution in cathodes for Li ion batteries [J]. Journal of Power Sources, 2003, 119-121(1): 770-773.
利用新颖的三层电极提高LiFePO4/C正极材料的电化学性能
肖政伟1,胡国荣2,杜 柯2,彭忠东2
1. 昆明理工大学 冶金与能源工程学院,昆明 650093;
2. 中南大学 冶金与环境学院,长沙 410083
摘 要:对常用的两层电极(活性材料层|集电极)进行改进,提出一种新颖的夹心状三层电极(导电材料层|活性材料层|集电极)以提高LiFePO4/C的电化学性能。充放电测试表明:相比两层电极,三层电极中LiFePO4/C表现出更优的倍率性能。循环伏安和电化学阻抗测试表明:相比两层电极,三层电极中LiFePO4/C材料中的Fe3+/Fe2+氧化还原电对表现出更快的氧化还原速度。更好的可逆性能以及更低的电荷转移阻抗。在三层电极中,活性材料层表层中与LiFePO4/C颗粒尺寸相当的孔洞被粒径小得多的乙炔黑微粒填充,形成LiFePO4/C颗粒间的导电连接,为暴露在电解液主体LiFePO4/C颗粒中的LiFePO4晶体提供更多运输电子到达或离开的路径。
关键词:锂离子电池;LiFePO4;正极构造;三层电极;电化学性能
(Edited by Chao WANG)
Foundation item: Project (2010ZCO51) supported by Natural Science Foundation of Yunnan Province; Project supported by Analysis and Testing Foundation (2009-041) and Starting Research Fund (14118245) from Kunming University of Science and Technology
Corresponding author: Zheng-wei Xiao; Tel/Fax: +86-871-5916662; E-mail:csuxiao@163.com
DOI: 10.1016/S1003-6326(13)62871-X
Abstract: As an improvement on the conventional two-layer electrode (active material layer|current collector), a novel sandwich-like three-layer electrode (conductive layer|active material layer|current collector) for cathode material LiFePO4/C was introduced in order to improve its electrochemical performance. LiFePO4/C in the three-layer electrode exhibited superior rate capability in comparison with that in the two-layer electrode in accordance with charge-discharge examination. Cyclic voltammetry and electrochemical impedance spectroscopy indicated that Fe3+/Fe2+ redox couple for LiFePO4 in the three-layer electrode displayed faster kinetics, better reversibility and much lower charge transfer resistance than that in the two-layer electrode in electrochemical process. For three-layer electrode, the holes in the surface of active material layer were filled by smaller acetylene black grains, which formed electrical connections and provided more pathways to electron transport to/from LiFePO4/C particles exposed to the bulk electrolyte.


