DOI: 10.11817/j.ysxb.1004.0609.2021-39543
镍基合金TLP扩散焊接头中二元共晶沉淀相Ni-M(B、Si、Zr和Hf)的性能
张永志1,张红魁2,陈捷狮2, 3, 4,尹志康2,张泽强2,杨明远2
(1. 中国航发上海商用航空发动机制造有限责任公司,上海 201306;
2. 上海工程技术大学 材料工程学院,上海 201620;
3. 上海市激光先进制造技术协同创新中心,上海 201620;
4. 上海交通大学 材料科学与工程学院,上海 200240)
摘要:采用基于密度泛函理论的第一性原理平面波赝势计算方法,计算了Ni-M(B、Si、Zr和Hf)二元共晶沉淀相(Ni2B、Ni3B、Ni3B2、Ni23B6、Ni3Si、Ni5Zr和Ni5Hf)的相稳定性、弹性常数、硬度及相应的电子结构。相稳定性计算结果表明,二元共晶沉淀相结构稳定性变化趋势由高到低的顺序为Ni3Si、Ni5Zr、Ni2B、Ni5Hf、Ni3B、Ni3B2、Ni23B6,这意味着降熔元素Si形成的Ni3Si沉淀相稳定性强于降熔元素B、Zr和Hf形成的沉淀相。弹性力学计算表明,Ni3B2表现为很强的各向异性,Ni2B、Ni3B和Ni3Si表现出一定的各向异性,而Ni23B6、Ni5Zr和Ni5Hf则表现为较强的各向同性。二元共晶沉淀相B/G值均大于1.75,这意味着它们为延性相。硬度计算的由小到大的小趋势为Ni3B2、Ni3B、Ni23B6、Ni2B、Ni5Zr、Ni3Si、Ni5Hf,其与各相中Ni 3d与B 2p,Si 3p,Zr 4d和Hf 5d的原子轨道杂化强度密切相关。
关键词:
二元共晶沉淀相;相稳定性;弹性常数;硬度;电子结构;第一性原理;
文章编号:1004-0609(2021)-01-0084-12 中图分类号:TG146.411 文献标志码:A
引文格式:张永志, 张红魁, 陈捷狮,等. 镍基合金TLP扩散焊接头中二元共晶沉淀相Ni-M(B、Si、Zr和Hf)的性能[J]. 中国有色金属学报, 2021, 31(1): 84-95. DOI: 10.11817/j.ysxb.1004.0609.2020-39543
ZHANG Yong-zhi, ZHANG Hong-kui, CHEN Jie-shi, et al. Properties of Ni-M (B, Si, Zr and Hf) binary eutectic precipitates in TLP diffusion joint of nickel-base alloy[J]. The Chinese Journal of Nonferrous Metals, 2021, 31(1): 84-95. DOI: 10.11817/j.ysxb.1004.0609.2020-39543
镍基合金具有较高的高温强度、蠕变强度和持久强度,良好的高温抗氧化和抗腐蚀能力,以及优异的抗疲劳性能和组织稳定性[1-5],广泛应用于制造工业燃气轮机涡轮叶片等热端部件,尤其镍基单晶高温合金是制造航空发动机涡轮叶片的首选材料[5-6]。随着涡轮叶片内部冷却结构设计复杂,仅采用精密铸造技术已不能满足要求,需与焊接技术相结合。然而,随着合金中难熔合金元素(W、Re、Ta、Mo)的添加,采用传统的熔焊方法,接头易出现组织偏析、杂晶、脆性相和低熔点共晶相等缺陷,并伴有热裂纹产生[7-10]。
瞬时液相扩散焊(Transient liquid phase, TLP)由DUVALL等[11]提出,此方法兼具高温钎焊和固相扩散焊的特点[7, 10],其过程是将一种与基体成分相似但添加降熔元素的中间层合金置于被连接基体之间,加热到一定温度和保温一段时间,降熔元素扩散到基体中,最终完成等温凝固。常用降熔元素有B、Si、Hf和Zr,降熔元素易与基体Ni在接头中出现共晶相,如采用B作为降熔元素对镍基高温合金TLP连接时,在接头中发现树枝状Ni2B[12]、块状Ni3B[13-15]、细小块状Ni3B2[12, 15-17]和针状Ni23B6[12, 16-17]共晶沉淀相。当采用降熔元素Si时,在接头中发现Ni3Si共晶[18]。而采用Hf和Zr时,在接头中同样发现Ni5Hf和Ni5Zr[19-20]。研究发现,这类共晶沉淀相的存在严重降低了焊接接头的综合力学性能,如硬度、抗拉强度、蠕变等性能[13-15]。虽然众多学者通过试验研究方法发现这类共晶沉淀相的存在,但关于其形成难易程度及力学性能鲜有报道。因此,本文采用第一性原理方法,系统地研究B、Si、Hf和Zr作为降熔元素与基体Ni形成的共晶沉淀相,即Ni2B、Ni3B、Ni3B2、Ni23B6、Ni3Si、Ni5Hf和Ni5Zr的结构稳定性和力学性能。从体系能量、电子结构和弹性性质等不同层面,探究二元共晶沉淀相的结构稳定性、力学性质和相互作用特性,旨在为镍基单晶合金TLP扩散焊中间层合金设计提供理论指导。
1 计算模型与方法
7种共晶沉淀相的晶胞模型如图1所示。其中Ni23B6( )、Ni3Si(
)、Ni3Si(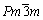 )、Ni5Hf(
)、Ni5Hf( )和Ni5Zr(
)和Ni5Zr( )为立方结构,Ni3B2(P4/mbm)和Ni2B (I4/mcm)为四方结构,而Ni3B(Pnma)为正交结构,其结构参数见表1。
)为立方结构,Ni3B2(P4/mbm)和Ni2B (I4/mcm)为四方结构,而Ni3B(Pnma)为正交结构,其结构参数见表1。
采用基于密度泛函理论(Density-functional theory)的CASTEP(Cambridge sequential total energy package)模块[21],运用平面波赝势方法,将离子势用赝势代替,电子波函数用平面波基函数线性展开,电子交换关联函数采用GGA(General gradient approximation)的PW91(Perdew and Wang)形式[22],分别采用超软赝势(Ultrasoft)[23]处理Ni(3d84s2)、B(2s22p1)、Si(3s23p2)、Zr(4d25s2)和Hf(5d26s2)的价电子。采用Broyden-Fletcher-Goldfarb-Shanno (BFGS)[24]算法对各种超晶胞模型进行几何优化,以获得局域最稳结构;平面波截断能ECu取350eV,各相的布里渊区K点积分用Monkhorst-Pack进行划分网格[25],其信息如表1所示。采用应力 -应变近似[26]计算弹性常数。在自洽计算(SCF)时应用Pulay密度混合法[27],自洽收敛条件设为:总能量小于 1×10-5 eV/atom,每个原子上的力低于0.3 eV/ 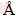 ,公差偏移小于1×10-3
,公差偏移小于1×10-3 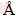 ,应力偏差小于0.05 GPa。
,应力偏差小于0.05 GPa。
2 结果与讨论
2.1 结构性能和稳定性
结构优化后的Ni2B、Ni3B、Ni3B2、Ni23B6、Ni3Si、Ni5Hf和Ni5Zr的晶格参数(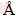 )、晶胞体积V (
)、晶胞体积V (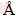 3)、生成焓△Hf (kJ/mol-atom)和结合能Ecoh (eV/atom)如表1所示[28-37]。结果表明,二元共晶沉淀相的晶格参数计算值与实验结果[28-34]差值均小于2%,并与理论计算值[35-36]相吻合,这说明了本文采用晶胞模型和计算方法精度可靠。
3)、生成焓△Hf (kJ/mol-atom)和结合能Ecoh (eV/atom)如表1所示[28-37]。结果表明,二元共晶沉淀相的晶格参数计算值与实验结果[28-34]差值均小于2%,并与理论计算值[35-36]相吻合,这说明了本文采用晶胞模型和计算方法精度可靠。
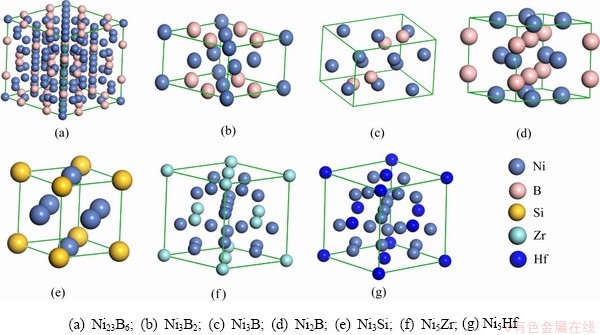
图1 晶体结构模型示意图
Fig. 1 Schematic diagrams of crystal structure model
表1 二元共晶沉淀相Ni-M(B、Si、Zr和Hf)的晶体参数(a, b, c)、晶胞体积V、生成焓△Hf和结合能Ecoh
Table 1 Lattice parameters (a, b and c), cell volume V, formation enthalpy △Hf and cohesive energy Ecoh of Ni-M (B, Si, Zr and Hf) binary eutectic precipitates

为了评价二元共晶沉淀相Ni-M(B、Si、Zr和Hf)在焊接接头中形成难易程度,对共晶沉淀相的生成焓△Hf和结合能Ecoh进行计算,公式如下[38]:
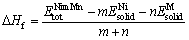 (1)
(1)
 (2)
(2)
式中: 为晶胞总能量;
为晶胞总能量; 和
和 分别为Ni在基态和自由态下原子的总能量;
分别为Ni在基态和自由态下原子的总能量;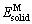 和
和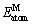 分别为降熔元素(MPD)在基态和自由态下原子的总能量;m、n分别表示Ni、M 原子在晶胞结构模型中原子个数。根据式(1)和(2),Ni-M(B、Si、Zr和Hf)的生成焓和结合能列于表1。晶体的结合能和结构稳定性密切相关,结合能是自由原子结合为晶体所释放的能量,即晶体分解成单个原子时对外界所做的功,根据能量最低原理,结合能越低其晶体结构越稳定。生成焓则表示金属的合金化能力,生成焓为负值时形成的化合物是稳定的,负值越负表示形成的化合物越稳定。通过对比各相的△Hf和Ecoh发现(见图2),二元共晶沉淀相(Ni2B、Ni3B、Ni3B2、Ni23B6、Ni3Si、Ni5Hf和Ni5Zr)生成焓值均为负值且结合能值大于0,表明各相结构是稳定且容易形成。结构稳定性变化趋势如下:Ni3Si>Ni5Zr>Ni2B>Ni5Hf>Ni3B>Ni3B2>Ni23B6,这表明与另外三种降熔元素B、Hf和Zr相比,降熔元素Si更容易形成二元沉淀相Ni3Si(-43.25 kJ/mol)。此外,Ni23B6、Ni3Si、Ni5Hf和Ni5Hf的生成焓分别为-21.71、-43.13、-33.75和-29.95 kJ/mol,其结果和实验值[33, 35-37]与理论计算[35-36]均相符,偏差均小于2%。
分别为降熔元素(MPD)在基态和自由态下原子的总能量;m、n分别表示Ni、M 原子在晶胞结构模型中原子个数。根据式(1)和(2),Ni-M(B、Si、Zr和Hf)的生成焓和结合能列于表1。晶体的结合能和结构稳定性密切相关,结合能是自由原子结合为晶体所释放的能量,即晶体分解成单个原子时对外界所做的功,根据能量最低原理,结合能越低其晶体结构越稳定。生成焓则表示金属的合金化能力,生成焓为负值时形成的化合物是稳定的,负值越负表示形成的化合物越稳定。通过对比各相的△Hf和Ecoh发现(见图2),二元共晶沉淀相(Ni2B、Ni3B、Ni3B2、Ni23B6、Ni3Si、Ni5Hf和Ni5Zr)生成焓值均为负值且结合能值大于0,表明各相结构是稳定且容易形成。结构稳定性变化趋势如下:Ni3Si>Ni5Zr>Ni2B>Ni5Hf>Ni3B>Ni3B2>Ni23B6,这表明与另外三种降熔元素B、Hf和Zr相比,降熔元素Si更容易形成二元沉淀相Ni3Si(-43.25 kJ/mol)。此外,Ni23B6、Ni3Si、Ni5Hf和Ni5Hf的生成焓分别为-21.71、-43.13、-33.75和-29.95 kJ/mol,其结果和实验值[33, 35-37]与理论计算[35-36]均相符,偏差均小于2%。
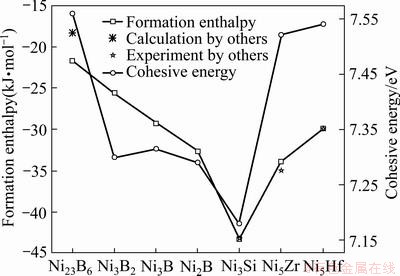
图2 二元共晶沉淀相Ni-M(B、Si、Zr和Hf)的生成焓和结合能
Fig. 2 Formation enthalpy and cohesive energy of Ni-M (B, Si, Zr and Hf) binary eutectic precipitates
2.2 力学性能
连接界面中形成的二元共晶沉淀相,因其本身脆而硬和各项异性的属性,往往成为接头界面裂纹的萌生处,严重降低了连接界面的综合性能,因此有必要对二元共晶沉淀相的力学性能进行研究。
2.2.1 力学稳定性
对于本文三类结构[39-41],其力学性能稳定性的限制条件如下所示:
立方结构(3个独立弹性常数C11、C12和C44)[39]:
C11>0,C44>0,C11>|C12|,C11+2C12>0 (3)
四方结构(6个独立弹性常数C11、C12、C23、C33、C44和C66)[40]:
Cij>0 (i=j=1, 3, 4, 6),C11>C12,C11+C33-2C13>0,
2C11+C33+2C12+4C13>0 (4)
正交结构(9个独立弹性常数C11、C12、C13、C22、C23、C33、C44、C55和C66)[41]:
Cij>0 (i=j=1, 2, 3, 4, 5, 6),
C11+ C22+C33+2(C12+C13+C23)>0,C11+C22-2C12>0,
C11+C33-2C13>0,C22+C33-2C33>0 (5)
各二元共晶沉淀相弹性常数Cij和弹性柔度矩阵Sij分别如表2和3所示。力学稳定性限制条件如式(3)~(5)所示,发现二元共晶沉淀相(Ni2B、Ni3B、Ni3B2、Ni23B6、Ni3Si、Ni5Hf和Ni5Zr)均满足力学性能稳定性限制条件,因此Ni2B、Ni3B、Ni3B2、Ni23B6、Ni3Si、Ni5Hf和Ni5Zr二元共晶沉淀相是力学稳定的。此外,Ni23B6,Ni3B, Ni2B和Ni3Si的弹性常数Cij均和理论值[35, 42]相近。
2.2.2 力学性能
表2 二元共晶沉淀相Ni-M(B、Si、Hf和Zr)的弹性常数
Table 2 Elastic constants of Ni-M (B, Si, Zr and Hf) binary eutectic precipitates
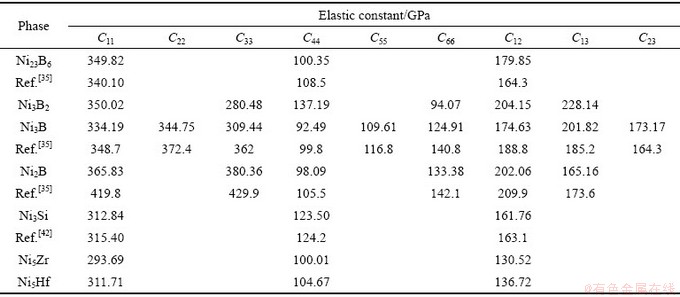
表3 二元共晶沉淀相Ni-M(B、Si、Hf和Zr)的弹性柔度常数
Table 3 Elastic compliance constants of Ni-M (B, Si, Hf and Zr) binary eutectic precipitates

利用Voigt(V)-Reuss(R)理论[43-45]计算了各相的体模量(B),剪切模量(G),弹性模量(E)和泊松比( )。
)。
立方结构时[43-45]:
 (6)
(6)
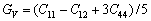 (7)
(7)
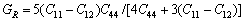 (8)
(8)
四方结构时[43-45]:
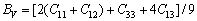 (9)
(9)
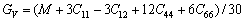 (10)
(10)
 (11)
(11)
 (12)
(12)
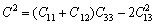 (13)
(13)
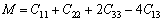 (14)
(14)
正交结构时[43-45]:
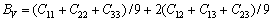 (15)
(15)
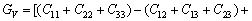
 (16)
(16)
 (17)
(17)
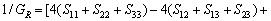
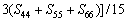 (18)
(18)
HILL[43]提出多晶体的实际值可以用Voigt方法和Reuss方法的弹性模量的算术平均值表示,即:
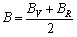 ,
,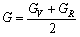 (19)
(19)
此外,弹性模量(E)和泊松比( )计算公式如下[43]:
)计算公式如下[43]:
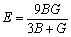 ,
,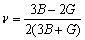 (20)
(20)
各相的体模量(B)、剪切模量(G)、弹性模量(E)和泊松比( ) 计算结果见表4。体模量(B)是反映材料抗体积变化能力的量度[46],由图3(a)可知,降熔元素B形成的二元共晶沉淀相与降熔元素Si、Zr和Hf形成的二元沉淀相相比,前者具有较高的体模量,表明Ni-B二元共晶沉淀相具有较强的抗断裂的能力。剪切模量(G)是衡量材料抵抗塑性变形能力的量度,通常与化学键的强度和方向性有密切关系[46],由图3(a)可知,二元共晶沉淀相的剪切模量趋势是: Ni2B>Ni3Si>Ni5Hf>Ni5Zr>Ni23B 6>Ni3B>Ni3B2,这表明二元沉淀相Ni2B和Ni3Si具有较强的方向性价键特性。弹性模量(E)是表征在弹性限度内材料抗拉或抗压的物理量,可作为衡量材料产生弹性变形难易程度的指标,通常其值越大,使材料发生一定变形的应力也越大[41],由图3(a)可知,降熔元素Hf形成的二元共晶沉淀相与降熔元素B、Si和Zr形成的二元共晶沉淀相相比,前者具有较低的杨氏模量,表明Ni5Hf共晶沉淀相比降熔元素B,Si和Zr形成的二元共晶沉淀相更容易发生变形。泊松比(
) 计算结果见表4。体模量(B)是反映材料抗体积变化能力的量度[46],由图3(a)可知,降熔元素B形成的二元共晶沉淀相与降熔元素Si、Zr和Hf形成的二元沉淀相相比,前者具有较高的体模量,表明Ni-B二元共晶沉淀相具有较强的抗断裂的能力。剪切模量(G)是衡量材料抵抗塑性变形能力的量度,通常与化学键的强度和方向性有密切关系[46],由图3(a)可知,二元共晶沉淀相的剪切模量趋势是: Ni2B>Ni3Si>Ni5Hf>Ni5Zr>Ni23B 6>Ni3B>Ni3B2,这表明二元沉淀相Ni2B和Ni3Si具有较强的方向性价键特性。弹性模量(E)是表征在弹性限度内材料抗拉或抗压的物理量,可作为衡量材料产生弹性变形难易程度的指标,通常其值越大,使材料发生一定变形的应力也越大[41],由图3(a)可知,降熔元素Hf形成的二元共晶沉淀相与降熔元素B、Si和Zr形成的二元共晶沉淀相相比,前者具有较低的杨氏模量,表明Ni5Hf共晶沉淀相比降熔元素B,Si和Zr形成的二元共晶沉淀相更容易发生变形。泊松比( )是用来评估材料抗剪切稳定性的参量,
)是用来评估材料抗剪切稳定性的参量, 值越大的材料塑性越好[41],由图3(b)可知,降熔元素B形成的二元共晶沉淀相与降熔元素Si、Zr和Hf形成的二元沉淀相相比,前者具有较高的泊松比,表明Ni-B二元共晶沉淀相具有较好的塑性。根据Pugh准则[46],材料的脆韧可用B/G值评判,其临界值为1.75。当B/G>1.75时,材料表现为塑性,当B/G<1.75否则为脆性;从表3b可看到各相的B/G值均大于1.75,表明各相均为塑性。另外,本文计算结果与理论结果[35-36, 42]也较好吻合。
值越大的材料塑性越好[41],由图3(b)可知,降熔元素B形成的二元共晶沉淀相与降熔元素Si、Zr和Hf形成的二元沉淀相相比,前者具有较高的泊松比,表明Ni-B二元共晶沉淀相具有较好的塑性。根据Pugh准则[46],材料的脆韧可用B/G值评判,其临界值为1.75。当B/G>1.75时,材料表现为塑性,当B/G<1.75否则为脆性;从表3b可看到各相的B/G值均大于1.75,表明各相均为塑性。另外,本文计算结果与理论结果[35-36, 42]也较好吻合。

图3 二元共晶沉淀相Ni-M(B、Si、Zr和Hf)的机械性能
Fig. 3 Mechanical properties of Ni-M(B, Si, Zr and Hf) binary eutectic precipitates
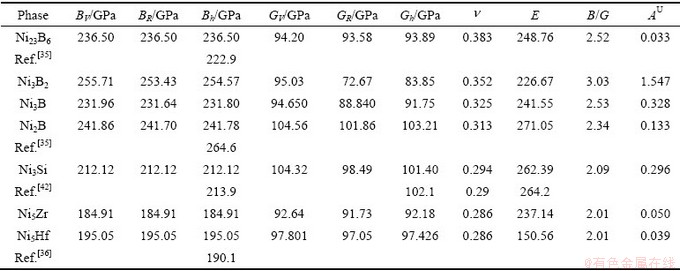
表4 二元共晶沉淀相Ni-M(B、Si、Zr和Hf)的体模量B,剪切模量G,弹性模量E,泊松比 和各项异性(AU)
和各项异性(AU)
Table 4 Bulk modulus B, Shear modulus G, elastic modulus E, Possion’s ratio  and Universal anisotropy index (AU) of Ni-M (B, Si, Zr and Hf) binary eutectic precipitates
and Universal anisotropy index (AU) of Ni-M (B, Si, Zr and Hf) binary eutectic precipitates
2.2.3 维氏硬度
对于有共价键的过渡金属多晶体材料,通常采用体模量和剪切模量来预测固有硬度;尽管体模量和剪切模量与硬度之间的关系并不十分准确,但从宏观参数得到的硬度还是可以了解材料硬度的控制因素,对了解连接界面硬度提供帮助。因此,以下分别采用Tian’s和Chen’s模型对各相硬度进行计算。
Tian’s半经验公式[47]:
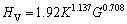 (21)
(21)
Chen’s半经验公式[48]:
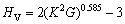 (22)
(22)
式中:K为G/B比值。根据式(21)~(22),各相的硬度计算结果见表4。从表4可知,两种半经验公式计算的结果趋势一致,Chen’s理论计算结果低于Tian’s结果。此外,降熔元素B形成的二元共晶沉淀相与降熔元素Si、Hf和Zr形成的二元沉淀相相比,具有较低的硬度,且Ni3B2相的硬度最低,这与Ni3B2相有较高的体模量与较低的剪切模量有关。
2.2.4 弹性各向异性
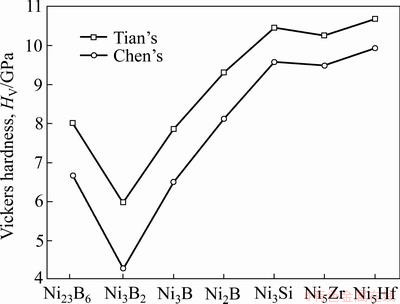
图4 维氏硬度对比
Fig. 4 Comparison of Vickers hardness calculation results
晶体的弹性各向异性因子在物理性能评价和预测中具有重要作用,可用各向异性指数(AU)衡量[49]:
 (23)
(23)
式(23)计算所得结果见表4。一般来说,AU值越大晶体的弹性各向异性程度越大。此外,晶体的弹性各向异性可利用三维曲面来表示,该曲面表明了晶体弹性特性与晶体学方向有关,不同结构的Ni-M(B、Si、Zr和Hf)二元共晶沉淀相的弹性模量的方向依赖关系定义如下[41]。
对于立方结构晶体,沿某一晶向的弹性模量可表示为
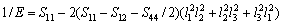 (24)
(24)
对于四方结构晶体,则有:
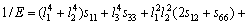
 (25)
(25)
对于正交结构晶体,则有:
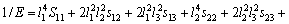
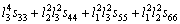 (26)
(26)
式中:Sij是单晶体弹性柔度系数;l1,l2,l3是晶向关于X,Y,Z轴的方向余弦。根据式(24)~(26)和表3结果,Ni-M(B、Si、Zr和Hf)二元共晶沉淀相弹性各项异性三维示意图如图5所示。三维示意图中的球形代表弹性各向同性,曲面背离球面的程度代表弹性各向异性程度。由图5可以发现Ni3B2相各相异性最大,这与Ni3B2相有较高的体模量和较低的剪切模量有一定关系。此外,各相的AU的计算值与三维(3D)曲面非常贴切。
2.3电子结构
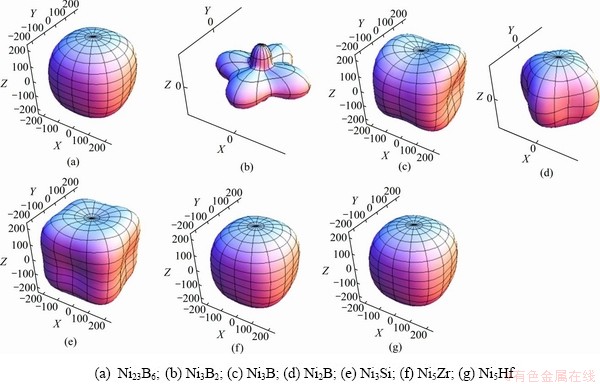
图5 二元共晶沉淀相Ni-M(B、Si、Hf和Zr)的弹性各向异性三维曲面图
Fig. 5 Elastic anisotropy three-dimensional surface of Ni-M(B, Si, Hf and Zr)binary eutectic precipitates
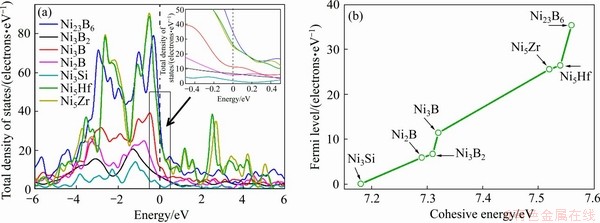
图6 二元共晶沉淀相Ni-M(B、Si、Hf和Zr)的总态密度(a)和费米能与结合能的关系(b)
Fig. 6 Total density of states(a) and relationship between Fermi level and cohesive energy(b) of Ni-M(B, Si, Hf and Zr) binary eutectic precipitates
为了进一步了解各相成键特性,分别计算了各相的电子结构,并且进一步揭示了各相的稳定性和力学性能与电子结构三者之间的关系。几何结构优化后,各相的总态密度(Total density of states,TDOS)计算结果见图6,其中能量值在0 eV处的垂直虚线表示费米能的位置。由图6(a)可发现,各相在费米能位置的TDOS值不为0,表明各相有一定的导电性能,即为金属材料。此外,费米能级的位置和费米能级处态密度的数值与材料的稳定性有很大关系,费米能级处态密度数值越低,材料结构越稳定。如图6(a)所示,各相在费米能级处态密度值大小关系为Ni3Si<Ni2B<Ni3B2<Ni3B<Ni5Zr<Ni5Hf<Ni23B6,表明Ni3Si结构最稳定,Ni23B6相对不稳定,结合前文结合能计算,如图6(b)所示,费米能级处态密度值与结合能呈正向关系。
由于各相的态密度主要有Ni 3d轨道贡献和部分B 2p,Si 3p,Zr 4d和Hf 5d轨道贡献,因此研究各相的分态密度(Partial density of states,PDOS)对于了解原子间成键特性具有重要价值。通常,原子之间杂化程度越大阴影面积越大,即原子之间共价键越强,材料表现出硬度越高。如图7所示的PDOS图,在费米能级左侧Ni 3d轨道分别与B 2p,Si 3p,Zr 4d和Hf 5d轨道发生3d 2p,3d3p,3d4d,3d5d不同程度的杂化(阴影部分),杂化关系为 Ni3B2<Ni3B<Ni23B6<Ni2B<Ni5Zr<Ni3Si<Ni5Hf,表明Ni5Hf相硬度最大,Ni3B2相硬度最小,其和维氏硬度计算结果一致。
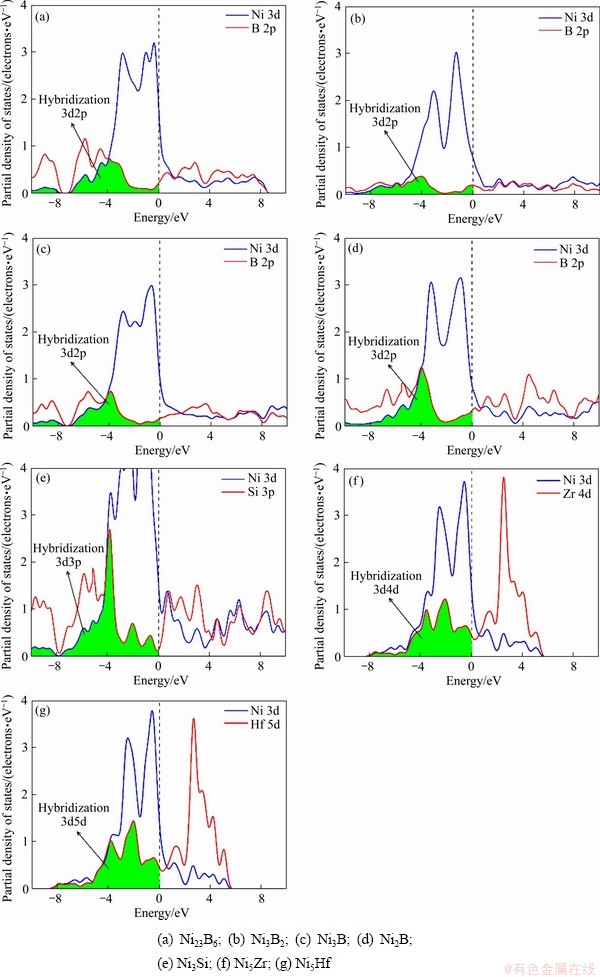
图7 二元共晶沉淀相Ni-M(B、Si、Zr和Hf)的分态密度
Fig. 7 Partial density of states of Ni-M(B, Si, Zr and Hf) binary eutectic precipitates
3 结论
1) 通过结构优化计算,Ni-M(B、Si、Zr和Hf)二元共晶沉淀相晶格常数与实验值及理论计算误差小于2%。生成焓和结合能结果表明,各二元共晶沉淀相均是稳定存在的,且Ni3Si合金化能力最强,即容易形成且结构稳定。
2) 弹性性质计算发现, Ni3B2表现为很强的各向异性,Ni2B、Ni3B和Ni3Si表现出一定的各向异性,而Ni23B6、Ni5Zr和Ni5Hf则表现为较强的各向同性。结合体模量、B/G和各向异性比较,二元共晶沉淀相力学上是稳定存在的且均为塑性相,其中Ni3B2相塑性最好但各向异性最大,Ni5Hf和Ni5Zr相塑性较差且各向异性较小。
3) 电子结构分析表明,费米能级处的TDOS值不为0,即各相表现为金属属性,并且TDOS值与结构越稳定计算结果存在对应关系。二元共晶沉淀相硬度关系为Ni3B2<Ni3B<Ni23B6<Ni2B<Ni5Zr<Ni3Si<Ni5Hf,其和各相中Ni 3d与B 2p,Si 3p,Zr 4d,Hf 5d的原子轨道杂化程度贡献密切相关。
REFERENCES
[1] BIAN H K, XU X D, LI Y P, et al. Regulating the coarsening of the γ′ phase in superalloys[J]. NPG Asia Materials, 2015, 7(8): 212-212.
[2] KOVARIK L, UNOCIC R R, LI J, et al. Microtwinning and other shearing mechanisms at intermediate temperatures in Ni-based superalloys[J]. Progress in Materials Science,2009, 54(6): 839-873.
[3] MASOUMI F, SHAHRIARI D, JAHAZI M, et al. Kinetics and mechanisms of gamma’ reprecipitation in a Ni-based superalloy[J]. Scientific reports, 2016, 6: 28650.
[4] SUN C, KIRK M, LI M, et al. Microstructure, chemistry and mechanical properties of Ni-based superalloy Rene N4 under irradiation at room temperature[J]. Acta Materialia, 2015, 95: 357-365.
[5] 田素贵, 朱新杰, 田 宁, 等. Ru对镍基单晶合金组织结构与蠕变行为的影响[J]. 中国有色金属学报, 2018, 28(2): 275-284.
TIAN Su-gui, ZHU Xin-jie, TIAN Ning, et al. Influence of element Ru on microstructure and creep behavior of single crystal nickel-based superalloy[J]. The Chinese Journal of Nonferrous Metals, 2018, 28(2): 275-284.
[6] CUI R J, HUANG Z H. Microstructual evolution and stability of second generation single crystal nickel-based superalloyDD5[J]. Transactions of Nonferrous Metals Society of China, 2016, 26(8): 2079-2085.
[7] CAVALIERE P, PANELLA F. Mechanical and microstructural behaviour of CMSX-4 Ni-based superalloy joined by capacitor discharge welding[J]. Journal of Materials Processing Technology, 2007, 183(2/3): 297-300.
[8] DYE D, CONLON K T, LEE P D, et al. Welding of single crystal superalloy CMSX-4: Experiments and modeling[J]. Superalloys, 2004: 485-491.
[9] OLA O T, OJO O A, WANJARA P, et al. A study of linear friction weld microstructure in single crystal CMSX-486 superalloy[J]. Metallurgical and Materials Transactions A, 2011, 43(3): 921-933.
[10] VITEK J M, BABU S S, PARK J W, et al. Analysis of stray grain formation in single-crystal nickel-based superalloy welds[J]. Superalloys, 2004: 459-465.
[11] DUVALL D S, OWCZARSKI W A, DF P. TLP bonding: a new method for joining heat resistant alloys[J]. Welding Journal, 1974, 53(4): 203-214.
[12] ZHANG H R, GHONEIM A, OJO O A. TEM analysis of diffusion brazement microstructure in a Ni3Al-based intermetallic alloy[J]. Journal of Materials Science, 2010, 46(2): 428-437.
[13] SHENG N C, LI B, LIU J D, et al. Influence of the substrate orientation on the isothermal solidification during TLP bonding single crystal superalloys[J]. Journal of Materials Science & Technology, 2014, 30(3): 213-216.
[14] SHENG N C, LIU J D, JIN T, SUN X F, HU Z Q. Wide gap TLP bonding a single-crystal superalloy: Evolution of the L/S interface morphology and formation of the isolated grain boundaries[J]. Metallurgical and Materials Transactions A, 2012, 44(4): 1793-1804.
[15] SUN Y, LIU J, LI B, et al. Microstructure evolution of single crystal superalloy DD5 joints brazed using AWS BNi-2 filler alloy[J]. Materials Research Innovations, 2014, 18(sup4): S4-341-S4-346.
[16] SHENG N C, LIU J D, JIN T, et al. Precipitation behaviors in the diffusion affected zone of TLP bonded single crystal superalloy joint[J]. Journal of Materials Science & Technology, 2015, 31(2): 129-134.
[17] 李晓红, 毛 唯, 钟群鹏, 等. DD3单晶合金TLP扩散焊接头组织及持久性能[J]. 焊接学报, 2012, 33(7): 1-4.
LI Xiao-hong, MAO Wei, ZHONG Qun-peng, et al. Microstructure and stress-rupture property of TLP diffusion bonded DD3 single crystal superalloy joints[J]. Transactions of the China Welding Institution, 2012, 33(7): 1-4.
[18] JALILIAN F, JAHZAI M, DREW R A L. Microstructural evolution during transient liquid phase bonding of Inconel 617 using Ni-Si-B filler metal[J]. Materials Science and Engineering A, 2006, 423(1/2): 269-281.
[19] ZHANG Y R, RUAN Z C. Microstructure and performance of Ni-Hf brazing filler alloy[J]. Acta Metallurgica Sinica B, 1990, 3(5): 335-340.
[20] ZHENG Y, ZHAO L, TANGRI K. Microstructure of Ni-10Co-8Cr-4W-13Zr alloy and its bonding behaviour for single-crystal nickel-base superalloy[J]. Journal of Materials Science,1993, 28(3): 823-829.
[21] CLARK S J, SEGALL M D, PICKARD C J, et al. First principles methods using CASTEP[J]. Journal of Crystallography-Crystalline Materials, 2005, 220(5/6): 567-570.
[22] PERDEW J P, CHEVARY J A, VOSKO S H, et al. Erratum: Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation[J]. Physical Review B, 1993, 48(7): 4978-4978.
[23] VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism[J]. Physical Review B, 1990, 41(11): 7892-7895.
[24] PFROMMER B G, COTE M, LOUIE S G, et al. Relaxation of crystals with the quasi-Newton method[J]. Journal of Computational Physics, 1997, 131(1): 233-240.
[25] MONKHORST H J, PACK J D. Special points for Brillouin-zone integrations[J]. Physical Review B, 1976, 13(12): 5188-5192.
[26] KARKI B B, STIXRUDE L, CRAIN J. Ab initio elasticity of three high-pressure polymorphs of silica[J]. Geophysical Research Letters, 1997, 24(24): 3269-3272.
[27] HAMMER B, HANSEN L B, NORSKOV J K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerh of functionals[J]. Physical Review B, 1999, 59(11): 7413-7421.
[28] IDZIKOWSKI B, SZAJEK A, Formation of the nanocrystalline cubic (Fe Ni)23 B6 phase in the nickel-rich Fe Ni Zr B alloys and its magnetic behavior[J].Journal of Optoelectronics and Advanced Materials, 2003, 5(1): 239-244.
[29] HU X B, ZHU Y L, SHENG N C, et al. The Wyckoff positional order and polyhedral intergrowth in the M3B2- and M5B3-type boride precipitated in the Ni-based superalloys[J]. Scientific Reports, 2014, 4: 7367.
[30] GUMENIUK R, BORRMANN H, LEITHE J A. Refinement of the crystal structures of trinickel boron Ni3B and tripalladium boron Pd3B[J]. Journal of Crystallography-New Crystal Structures, 2006, 221(4): 425-426.
[31] CHAKOUMAKOS B C, PARANTHAMAN M. Neutron powder diffraction study of the superconducting quaternary intermetallic compound YNi2B2C[J]. Physica C (Superconductivity), 1994, 227(1/2): 143-150.
[32] ACKERBAUER S, KRENDELSBERGER N, WEITZER F, HIEBL K, SCHUSTER J C. The constitution of the ternary system Fe-Ni-Si[J]. Intermetallics, 2009, 17(6): 414-420.
[33] FOREY P, GLIMOIS J L, FERON J L. Study on the structure of ternary alloy (Ni1-xCux)5Zr[J]. Journal of the Less Common Metals, 1986, 124(1/2): 21-27.
[34] KIRKPATRICK M E, LARSEN W L. Phase relationships in the nickel-zirconium and nickel-hafnium alloy systems[J]. Transactions of the American Society for Metals, 1961, 54: 580-590.
[35] KONG Y, XIONG W, GUO H B, et al. Elastic and thermodynamic properties of the Ni-B system studied by first-principles calculations and experimental measurements[J]. Calphad, 2010, 34(2): 245-251.
[36] RADAKOVIC J, CIRIC K, BELOSEVIC C J, et al. First-principles study of the thermodynamic properties and electronic structure of compounds from Hf-Ni phase[J]. Computational Materials Science, 2010, 49(1): 55-59.
[37] HENAFF M P, COLINET C, PASTUREL A, et al. Study of the enthalpies of formation and crystallization in the system Zr-Ni[J]. Journal of Applied Physics, 1984, 56(2): 307-310.
[38] KELLOU A, FERAOUN H I, GROSDIDIER T, et al. Energetics and electronic properties of vacancies, anti-sites, and atomic defects (B, C, and N) in B2-FeAl alloys[J]. Acta Materialia, 2004, 52(11):3263-3271.
[39] PATIL S K R, KHARE S V, TUTTLE B R, et al. Mechanical stability of possible structures of PtN investigated using first-principles calculations[J]. Physical Review B, 2006, 73(10).
[40] BORN M, HUANG K, LAX M. Dynamical theory of crystal lattices[J]. American Journal of Physics, 1955, 23(7): 474-474.
[41] NYE J F, LINDSAY R B. Physical properties of crystals: Their representation by tensors and matrices[J]. Physics Today, 1957, 10(12): 26-26.
[42] CAO Y, ZHU J C, LIU Y, et al. First-principles studies of the structural, elastic, electronic and thermal properties of Ni3Si[J]. Computational Materials Science, 2013, 69: 40-45.
[43] HILL R. The elastic behaviour of a crystalline aggregate[J]. Proceedings of the Physical Society Section A, 1952, 65(5): 349-354.
[44] HUANG Z C, FENG J, PAN W. Theoretical investigations of the physical properties of zircon-type YVO4[J]. Journal of Solid State Chemistry, 2012, 185: 42-48.
[45] PANDA K, CHANDRAN K. First principles determination of elastic constants and chemical bonding of titanium boride (TiB) on the basis of density functional theory[J]. Acta Materialia, 2006, 54(6): 1641-1657.
[46] PUGH S F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals[J]. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science, 1954, 45(367): 823-843.
[47] TIAN Y J, XU B, ZHAO Z S. Microscopic theory of hardness and design of novel superhard crystals[J]. International Journal of Refractory Metals and Hard Materials, 2012, 33: 93-106.
[48] CHEN X Q, NIU H Y, LI D Z, et al. Modeling hardness of polycrystalline materials and bulk metallic glasses[J]. Intermetallics, 2011, 19(9): 1275-1281.
[49] RANGANATHAN S I, OSTOJA-STARZEWSKI M. Universal elastic anisotropy index[J]. Physical Review Letters, 2008, 101(5): 055504.
Properties of Ni-M (B, Si, Zr and Hf) binary eutectic precipitates in TLP diffusion joint of nickel-base alloy
ZHANG Yong-zhi1, ZHANG Hong-kui2, CHEN Jie-shi2, 3, 4, YIN Zhi-kang2, ZHANG Zhe-qiang2, YANG Ming-yuan2
(1. AECC Shanghai Commercial Aircraft Engine manufacturing Co., Ltd., Shanghai 201306, China;
2. School of Materials Engineering, Shanghai University of Engineering Science, Shanghai 201602, China;
3. Shanghai Collaborative Innovation Center of Laser Advanced Manufacturing Technology, Shanghai 201620, China;
4. School of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China)
Abstract: A systematic investigation concerned with phase stability, elastic properties, hardness and relevant electronic structure of Ni-M binary eutectic precipitates (Ni2B, Ni3B, Ni3B2, Ni23B6, Ni3Si, Ni5Zr and Ni5Hf) was carried out using first principles calculations. The calculated results show that the change trend of the structural stability follows Ni3Si>Ni5Zr>Ni2B>Ni5Hf>Ni3B>Ni3B2>Ni23B6, indicating that the stability of Ni3Si phase using Si as melting-point depressant is stronger than that using B, Zr and Hf as melting-point depressants. The calculated elastic constants indicate that Ni3B2 is significantly anisotropic, and Ni2B, Ni3B and Ni3Si exhibit some anisotropy, while Ni23B6, Ni5Zr and Ni5Hfshow a relatively isotropic character. The ratios of bulk modulus (B) to shear modulus (G) are more than 1.75 for all the Ni-M binary eutectic precipitates in this research, indicating their ductile nature. The trend of Vickers hardness of these Ni-M binary eutectic precipitates is as Ni3B2<Ni3B<Ni23B6<Ni2B<Ni5Zr<Ni3Si<Ni5Hf, and the strength of Ni 3d, B 2p, Si 3p, Zr 4d and Hf 5d hybrid orbitals were found to play important roles in the hardness of these compounds.
Key words: binary eutectic precipitates; phase stability; elastic constant; hardness; electronic structure; first principles
Foundation item: Project(51805316) supported by the National Natural Science Foundation of China; Project (2019M651491) supported by China Postdoctoral Science Foundation; Project(18YF1424900) supported by Shanghai Sailing Program; Project(18511108600) supported by Shanghai Science and Technology Committee Innovation Grant
Received date: 2019-09-08; Accepted date: 2019-11-25
Corresponding author: CHEN Jie-shi; Tel: +86-21-67791474; E-mail: cjshbb@sjtu.edu.cn
(编辑 龙怀中)
基金项目:国家自然科学基金资助项目(51805316);中国博士后科学基金资助项目(2019M651491);上海市青年科技英才扬帆计划资助项目(18YF1424900);上海市科学技术委员会科研计划资助项目(18511108600)
收稿日期:2019-09-08;修订日期:2019-11-25
通信作者:陈捷狮,副教授,博士;电话:021-67791474;E-mail:cjshbb@sjtu.edu.cn


