Trans. Nonferrous Met. Soc. China 27(2017) 562-568
Influence of chloride salts on hydrogen generation via hydrolysis of MgH2 prepared by hydriding combustion synthesis and mechanical milling
Shu LI, De-yu GAN, Yun-feng ZHU, Ya-na LIU, Ge ZHANG, Li-quan LI
College of Materials Science and Engineering, Nanjing Tech University, Nanjing 210009, China
Received 5 January 2016; accepted 8 July 2016
Abstract:
The effects of chloride salts (NaCl, MgCl2 and NH4Cl) on the hydrolysis kinetics of MgH2 prepared by hydriding combustion synthesis and mechanical milling (HCS+MM) were discussed. X-ray diffraction (XRD) analyses show that high-purity MgH2 was successfully prepared by HCS. Hydrolysis performance test results indicate that the chloride salt added during the milling process is favorable to the initial reaction rate and hydrogen generation yield within 60 min. A MgH2-10% NH4Cl composite exhibits the best performance with the hydrogen generation yield of 1311 mL/g and a conversion rate of 85.69% in 60 min at room temperature. It is suggested that the chloride salts not only play as grinding aids in the milling process, but also create fresh surface of reactive materials, favoring the hydrolysis reaction.
Key words:
MgH2; hydrogen generation yield; hydrolysis reaction; chloride salts; hydriding combustion synthesis; mechanical milling;
1 Introduction
Hydrogen source technology is one of the key technologies for the popularization and application of hydrogen fuel cells, especially in new energy vehicles and mobile power. At present, the commonly used on-board hydrogen source technologies are the high pressure gaseous hydrogen storage and cryogenic liquid hydrogen storage. However, the transportation cost is high and the safety is low [1], which hinder the practical application.
In recent years, hydrogen generation technologies of some cheap active metals and their hydrides have developed rapidly, such as Al [2], Mg [3], MgH2 [4-6]. The theoretical hydrogen generation by the hydrolysis of MgH2 is higher, which can reach 1700 mL/g. Considering the water produced by proton exchange membrane fuel cells can be reused and the theoretical hydrogen generation of the system can reach 15.2% (mass fraction) [7]. Therefore, many scientists have made a lot of research on the hydrolysis of MgH2.
The hydrolysis of MgH2 can react in the mild environment. However, the insoluble by-product Mg(OH)2 will cover the unreacted material, leading to the hydrolysis rate reduction. The hydrogen generation can be significantly improved when MgH2 reacts with acid solutions [5]. However, equipment will be corroded by acidic solution, which is not beneficial for the practical application.
Mechanical milling (MM) can effectively improve the hydrogen generation performance of MgH2. It is attributed to the formation of nanocrystalline MgH2 during MM process [8-10]. HUOT et al [11] received nanograins by milling the commercial MgH2 for 20 h. The hydrogen generation yield and the conversion rate got to 1020 mL/g and 60%, respectively, which were 2.5 times those of unmilled MgH2 [11]. TESSIER et al [12] mixed MgH2 with CaH2 during the milling process, in order to improve the dynamic performance of hydrogen generation by MgH2. They found that conversion rate of 80% could be obtained for 10 h milled MgH2-20%CaH2 (mole fraction) in 0.5 h, and the hydrogen generation efficiency was improved obviously [12]. However, it is not conducive to the development of cheap hydrogen source for the addition of expensive CaH2. Although the improvement of dynamic performance is significant, there is still a big gap to practical requirements.
The studies above all chose commercial MgH2 as raw material, and the cost was high. We have prepared high capacity and high activity MgH2 by hydriding combustion synthesis (HCS) [13,14]. ZHAO et al [6] achieved 1635 mL/g hydrogen from the hydrolysis of MgH2 prepared by HCS+MM in MgCl2 solution at 303 K. Chloride salts are reported to be beneficial for the hydrolysis kinetics of Mg-based material. This is mainly due to the brittleness of chloride salts, which can generate many scrappy particles in the milling process and increase the specific area of the powder [15-17]. However, the hydrogen generation performance of HCS MgH2 milled with chloride salts has not been studied.
In this work, the HCS products were ball milled with various chloride salts (NaCl, MgCl2 and NH4Cl) in order to further improve the hydrogen generation performance. The influences of chloride salt type and their additive amounts on the hydrolysis performance were discussed. The best performance was obtained with a composite of HCS MgH2-10%NH4Cl milled for 300 min, with the hydrogen generation amount of 1311 mL/g and a conversion rate of 85.69% in 60 min at room temperature.
2 Experimental
2.1 Preparation of samples
Mg powder (99.9% in purity and <45 μm in diameter), Ni (99.9% in purity and 2-3 μm in diameter), NaCl (AR, 99.5% in purity), MgCl2 (AR, 99% in purity), NH4Cl (AR, 99.5% in purity) were commercially gotten. All the samples were placed in a glove box filled with argon atmosphere in a circulation system.
Mg-based hydride was prepared by HCS as described in our previous work [13]. Mg powder and Ni powder were mixed with the mole ratio of 99:1 (Mg99Ni) for preparing the high content of MgH2. The powders were mixed in acetone by ultrasonic homogenizer, and then were dried in a drier at 326 K. The dried powders were directly used to prepare MgH2 by HCS method. The powders were heated to 853 K with a 10 K/min heating rate under 2 MPa hydrogen atmosphere and then held at this temperature for 1 h to make Mg and Ni totally transform into Mg2Ni. The powders were cooled down and held at 613 K for 10 h. Afterwards, the sample was cooled down to room temperature.
HCS products and various anhydrous chloride salts (NaCl, MgCl2 and NH4Cl) were mechanically milled to prepare the composites of MgH2-x%NaCl, MgH2- x%MgCl2 and MgH2-x%NH4Cl. MM was performed in a planetary ball mill (QM-3SP2) with a powder to ball mass ratio of 1:40 at 400 r/min for 300 min.
2.2 Hydrogen production performance test
The hydrolysis reaction was performed in a 100 mL flask with three openings: a water inlet, a hydrogen outlet and an opening sealed with a plug. The hydrogen was collected by an inverted cylinder filled with 200 mL water. The rubber tube was used to connect the hydrolysis reactor and the measuring cylinder. 0.1 g powder activated by MM and 10 mL distilled water were used in each hydrolysis reaction. Each test was repeated three times to confirm the precision of the measurement (±5%).
2.3 Microstructure characterization
The crystal structure and the phase composition of the samples were analyzed by X-ray diffraction (XRD) with Cu Kα radiation (40 kV and 35 mA) using an ARL X’TRA diffractometer. The average crystallite sizes of the samples were estimated by Scherrer formula [18]:
D=kλ/(βcosθ) (1)
where D, k, β, θ and λ represent respectively average grain size, shape factor, half-height width of the diffraction peak, Bragg diffraction angle and the wavelength of the incident radiation.
Scanning electron microscopy (SEM) was used to observe the microstructure of the samples. The elemental distribution of the samples was analyzed by energy dispersive X-ray spectroscopy (EDS, EDAX Inc.).
3 Results and discussion
Figure 1 displays the XRD pattern of Mg-based hydride prepared by HCS. It can be seen that the HCS product is composed of three phases: the main phase MgH2, the minor phases Mg2NiH4 and Mg. The presence of Mg phase indicates that the hydrogenation of Mg during the HCS process is not completed. In addition, it also indicates that the main and secondary hydrolysis reactions are the hydrolysis of MgH2 and Mg, respectively.
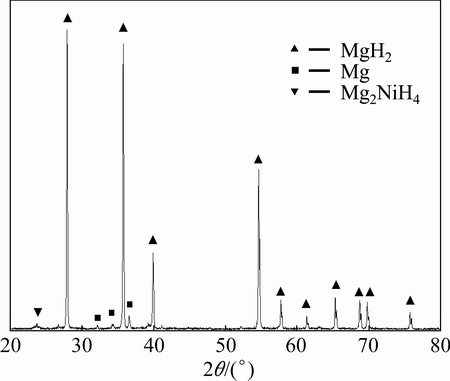
Fig. 1 XRD pattern of Mg-based hydride prepared by HCS
It has been reported that the addition of chloride salts during the milling process has significant impact on the hydrogen generation performance of Mg [15]. In order to improve the hydrogen generation performance of the HCS MgH2, various chloride salts were added in the milling process of MgH2. Hydrogen generation curves of MgH2 with different addition types of chloride salt prepared by HCS+MM are shown in Fig. 2. The hydrogen generation yield via the hydrolysis of MgH2 with 3% NaCl, MgCl2 and NH4Cl was studied. When the HCS MgH2 reacted with pure water, the hydrogen generation yield reached 710 mL/g within 60 min, which was higher than that from the hydrolysis of commercial MgH2 [19]. Chloride salts can improve the initial reaction rate and the hydrogen generation yield compared with MgH2 alone. It can be seen that, MgH2-3%NH4Cl shows the best performance with the hydrogen generation yield of 1072 mL/g within 60 min.
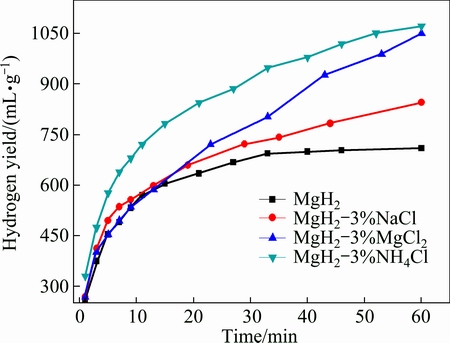
Fig. 2 Hydrogen generation curves of MgH2 with different addition types of chloride salt prepared by HCS+MM
3.1 Effect of NaCl additon
Figure 3 shows hydrogen generation curves of MgH2 with different addition amounts of NaCl prepared by HCS+MM. As can be seen in Fig. 3, the hydrogen generation yield within 60 min increases with increasing the amount of NaCl. It is worth noting that with increasing the amount of NaCl from 5% to 10%, the hydrogen generation yields are the same. The possible reasons may be as follows:the hydrolysis reaction rate of the MgH2-10%NaCl system in the initial reaction is more violent than that of the MgH2-5%NaCl system. Accordingly, a large amount of the in situ generated magnesium hydroxide covers the surface of the reactive material, preventing the reactive substance from continuous hydrolyzing. Therefore, the hydrogen yield is only 989 mL/g in this case. Hydrogen generation yield and conversion rate of MgH2-x%NaCl within 60 min are listed in Table 1. The conversion rate of MgH2-3%NaCl is raised to 51.24%, the MgH2-5%NaCl (61.24%) and the MgH2-10%NaCl (64.64%) samples also show better performance. The hydrogen generation yields of MgH2- 3%NaCl, MgH2-5%NaCl and MgH2-10%NaCl are 845, 989 and 989 mL/g, which are higher than 710 mL/g (without NaCl addition). It is evident that NaCl can improve the hydrogen generation yield and conversion rate of HCS products. In addition, the hydrolysis kinetics of MgH2 improves with increasing the content of NaCl, especially within the first 30 min. The reasons for this phenomenon may be that the efficiency of ball milling could be improved with the addition of NaCl in the ball milling process as a grinding aid, leading to grain refinement. Besides, the added NaCl is easy to be crushed into small particles and then covers the powder surface. The tiny NaCl particles are dissolved in water during the hydrolysis reaction, which makes more reactive material be exposed to the water. Therefore, the hydrogen generation performance in the initial stage of the system is improved.
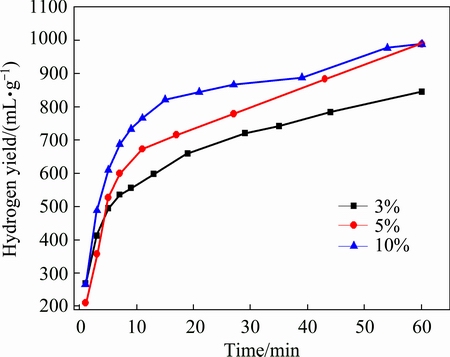
Fig. 3 Hydrogen generation curves of MgH2 with different addition amounts of NaCl prepared by HCS+MM
Table 1 Hydrogen generation yield and conversion rate of MgH2-x%NaCl within 60 min
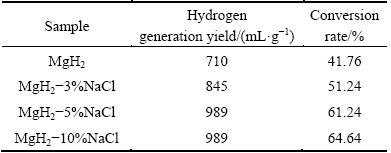
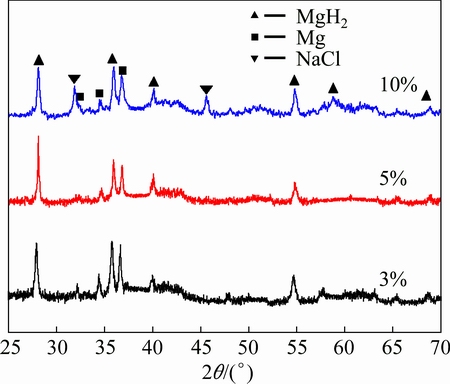
Fig. 4 XRD patterns of MgH2 with different addition amounts of NaCl prepared by HCS+MM
Figure 4 shows the XRD patterns of MgH2 with different addition amounts of NaCl prepared by HCS+MM. There is no new phase and MgH2 is still the main phase when MgH2 is milled with NaCl for 300 min, the diffraction peaks of MgH2 are obviously broadened. NaCl phase was not found in the XRD pattern when the amount of added NaCl is 3% and 5%. It could be explained that the amount of added NaCl is little and the NaCl phase becomes nano level during the milling process, being merged in the background. It can be estimated by Scherrer formula that the average grain sizes of MgH2 in the samples are 37, 35 and 30 nm for MgH2-3%NaCl, MgH2-5%NaCl and MgH2-10%NaCl, respectively. The results prove that the grain size of the products decreases with increasing the amount of NaCl during the ball milling process. SEM images of MgH2-x%NaCl prepared by HCS+MM and the EDS mapping for Na shown in Fig. 5 confirm that all products are small and uniform in particle size. NaCl is beneficial to decrease the agglomeration obviously, which is in accordance with the publications [20,21].
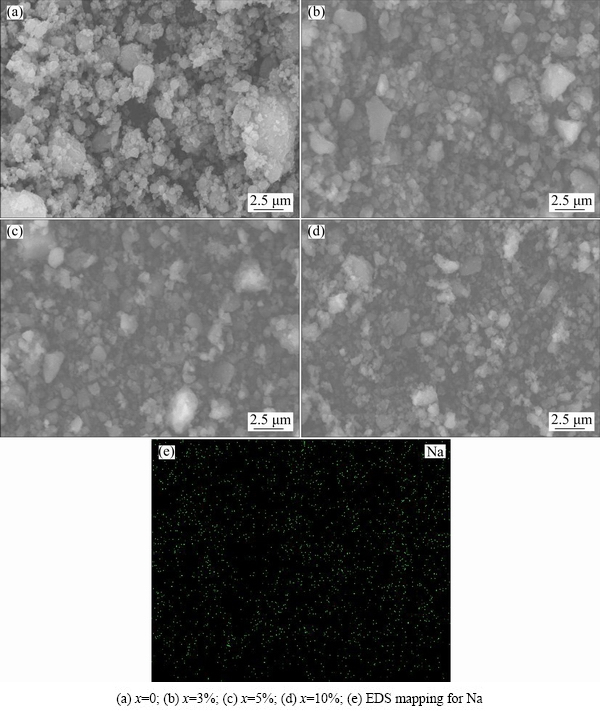
Fig. 5 SEM images of MgH2-xNaCl prepared by HCS+MM
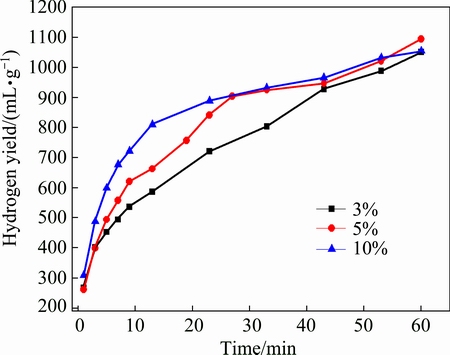
Fig. 6 Hydrogen generation curves of MgH2 with different addition amounts of MgCl2 prepared by HCS+MM
Table 2 Hydrogen generation yield and conversion rate of MgH2-xMgCl2 within 60 min

3.2 Effect of MgCl2 addition
Figure 6 shows the hydrogen generation curves of MgH2 with different addition amounts of MgCl2 prepared by HCS+MM. It can be seen that the hydrogen generation yield in the initial reaction increases with adding more MgCl2. However, the hydrogen generation yield is the highest for MgH2-5%MgCl2. Table 2 shows the hydrogen generation yield and the conversion rate of MgH2-xMgCl2 within 60 min. The hydrogen generation yield of MgH2-3%MgCl2 reaches 1051 mL/g, showing better hydrogen generation performance than MgH2-3%NaCl. When the amount of MgCl2 increases to 5%, the hydrogen generation yield reaches 1094 mL/g and the conversion rate is 67.74%. The experimental results reveal that there is no significant improvement in the hydrogen generation yield. However, the addition of MgCl2 has a significant effect on the initial dynamic performance of the hydrolysis reaction. A reasonable explanation is proposed: on one hand, the exothermic enthalpy of MgCl2 dissolution is much larger than that of NaCl (MgCl2: 155 kJ/mol; NaCl: 5 kJ/mol) [22]. The released heat makes the local temperature rise, which is beneficial for the local reaction. However, the temperature change is not obvious in the whole reaction system. On the other hand, magnesium ion is favorable to promote the hydrogen generation performance. This could be explained by the lower pH value of MgCl2 with higher molarity of Mg2+ according to Eq. (2). The dense magnesium hydroxide is more easily broken with decreasing the pH value, which attributes to the full hydrolysis.
lg[Mg2+]=16.95-2pH (2)
3.3 Effect of NH4Cl addition
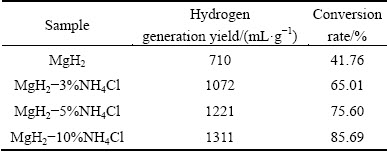
Figure 7 presents the hydrogen generation curves of MgH2 with different addition amounts of NH4Cl prepared by HCS+MM. The hydrogen generation rate in initial reaction and the hydrogen generation yield within 60 min both increase with increasing the content of NH4Cl. Table 3 shows the hydrogen generation yield and the conversion rate of MgH2-x%NH4Cl within 60 min. The hydrogen generation yield reaches 1311 mL/g and the conversion rate is 85.69% for MgH2-10%NH4Cl. The results represent that NH4Cl improves the hydrolysis performance of MgH2 significantly. This is probably because the addition of NH4Cl can not only effectively refine the MgH2 particles, but also provide strong acidity of the solution, which can dissolve the Mg(OH)2 passivation layer more effectively [23,24].
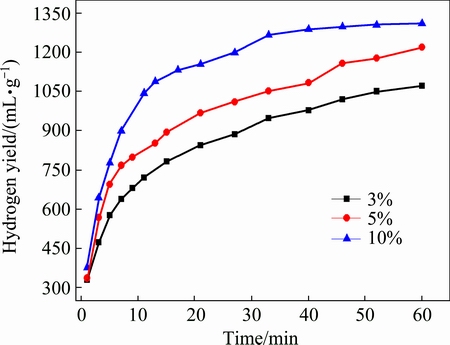
Fig. 7 Hydrogen generation curves of MgH2 with different addition amounts of NH4Cl prepared by HCS+MM
Table 3 Hydrogen generation yield and conversion rate of MgH2-xNH4Cl within 60 min
In summary, the hydrogen generation performance of MgH2 can be effectively improved by milling with chloride salts (NaCl, MgCl2 and NH4Cl). The same functions of adding the three chloride salts are proposed: 1) they play as grinding aids in the milling process, 2) salts dissolution make more fresh surface of MgH2 contacting with the water, 3) Cl- can break the Mg(OH)2 layer through a pit corrosion process. But adding the same amount of chloride salt, the samples exhibit different hydrogen generation performance. Among them, NH4Cl added during ball milling process shows the best promotion effect in the hydrogen generation. The hydrogen generation yield of MgH2-10%NH4Cl gets to 1311 mL/g and the conversion rate is 85.69% in 60 min. The high hydrogen yield for NH4Cl is due to the strong affinity between NH4+ and OH- [25].
4 Conclusions
1) The hydrogen generation by hydrolysis of MgH2 can be improved by adding chloride salts (NaCl, MgCl2 and NH4Cl) during the milling process.
2) The grain size of the MgH2-xNaCl decreases with increasing the amount of NaCl during the ball milling process. The average grain sizes of MgH2 in the samples are 37, 35 and 30 nm for MgH2-3%NaCl, MgH2-5%NaCl and MgH2-10%NaCl, respectively.
3) The promotion of NH4Cl on the hydrolysis kinetics is greater than the other two kinds of chloride salts. MgH2-10%NH4Cl milled for 300 min shows the best hydrogen production performance, with the hydrogen generation yield of 1311 mL/g and a conversion rate of 85.69% in 60 min at room temperature.
References
[1] TAYEH T, AWAD A S, NAKHL M, ZAKHOUR M, SILVAIN J F, BOBET J L. Production of hydrogen from magnesium hydrides hydrolysis [J]. International Journal of Hydrogen Energy, 2014, 39: 3109-3117.
[2] DAI Hong-bin, MA Guang-lu, XIA Hai-jie, WANG Ping. Reaction of aluminium with alkaline sodium stannate solution as a controlled source of hydrogen [J]. Energy & Environmental Science, 2011, 4: 2206-2212.
[3] UAN J Y, YU S H, LIN M C, CHEN L F, LIN H I. Evolution of hydrogen from magnesium alloy scraps in citric acid-added seawater without catalyst [J]. International Journal of Hydrogen Energy, 2009, 34: 6137-6142.
[4] KOJIMA Y, SUZUKI K I, KAWAI Y. Hydrogen generation by hydrolysis reaction of magnesium hydride [J]. Journal of Materials Science, 2004, 39: 2227-2229.
[5] HIRAKI T, HIROI S, AKASHI T, OKINAKA N, AKIYAMA T. Chemical equilibrium analysis for hydrolysis of magnesium hydride to generate hydrogen [J]. International Journal of Hydrogen Energy, 2012, 37: 12114-12119.
[6] ZHAO Ze-lun, ZHU Yun-feng, LI Li-quan. Efficient catalysis by MgCl2 in hydrogen generation via hydrolysis of Mg-based hydride prepared by hydriding combustion synthesis [J]. Chemical Communications, 2012, 48: 5509-5511.
[7] DENG Zhen-yan, FERREIRA J M F, SAKKA Y. Hydrogen generation materials for portable applications [J]. Journal of the American Ceramic Society, 2008, 91: 3825-34.
[8] SHAHI R R, BHATNAGAR A, PANDEY S K, DIXIT V, SRIVASTAVA O N. Effects of Ti-based catalysts and synergistic effect of SWCNTs-TiF3 on hydrogen uptake and release from MgH2 [J]. International Journal of Hydrogen Energy, 2014, 39: 14255-14261.
[9] SHAHI R R, TIWARI A P, SHAZ M A, SRIVASTAVA O N. Studies on de/rehydrogenation characteristics of nanocrystalline MgH2 co-catalyzed with Ti, Fe and Ni [J]. International Journal of Hydrogen Energy, 2013, 38: 2778-2784.
[10] LIU Guang, WANG Yi-jing, JIAO Li-fang, YUAN Hua-tang. Understanding the role of few-layer graphene nanosheets in enhancing the hydrogen sorption kinetics of magnesium hydride [J]. ACS Applied Materials & Interfaces, 2014, 6: 11038-11046.
[11] HUOT J, LIANG G, SCHULZ R. Magnesium-based nanocomposites chemical hydrides [J]. Journal of Alloys and Compounds, 2003, 353: L12-L5.
[12] TESSIER J P, PALAU P, HUOT J, SCHULZ R, GUAY D. Hydrogen production and crystal structure of ball-milled MgH2-Ca and MgH2-CaH2 mixtures [J]. Journal of Alloys and Compounds, 2004, 376: 180-185.
[13] LIU Xiao-feng, ZHU Yun-feng, LI Li-quan. Hydrogen storage properties of Mg100-xNix (x=5, 11.3, 20, 25) composites prepared by hydriding combustion synthesis followed by mechanical milling (HCS+MM) [J]. Intermetallics, 2007,15: 1582-1588.
[14] TAN Ya-jun , ZHU Yun-feng, LI Li-quan. Excellent catalytic effects of multi-walled carbon nanotube supported titania on hydrogen storage of a Mg-Ni alloy [J]. Chemical Communications, 2015, 51: 2368-2371.
[15] WANG Shuang, SUN Li-xian, XU Fen, JIAO Cheng-li, ZHANG Jian, ZHOU Huai-ying, HUANG Feng-lei. Hydrolysis reaction of ball-milled Mg-metal chlorides composite for hydrogen generation for fuel cells [J]. International Journal of Hydrogen Energy, 2012, 37: 6771-6775.
[16] GROSJEAN M,  L. Hydrolysis of Mg-salt and MgH2-salt mixtures prepared by ball milling for hydrogen production [J]. Journal of Alloys and Compounds, 2006, 416: 296-302.
L. Hydrolysis of Mg-salt and MgH2-salt mixtures prepared by ball milling for hydrogen production [J]. Journal of Alloys and Compounds, 2006, 416: 296-302.
[17] LIU Yong-gan, WANG Xin-hua, DONG Zhao-hui, LIU Hai-zhen, LI Shou-quan. Hydrogen generation from the hydrolysis of Mg powder ball-milled with AlCl3 [J]. Energy, 2013, 53: 147-152.
[18] DORSET D L. X-ray diffraction: A practical approach [M]. New York: Elsevier, 1998.
[19] HU L X, WANG E D. Hydrogen generation via hydrolysis of nanocrystalline MgH2 and MgH2-based composites [J]. Transactions of Nonferrous Metals Society of China, 2005, 15: 965-970.
[20] GROSJEAN M H, ZIDOUNE M,  L. Hydrogen production from highly corroding Mg-based materials elaborated by ball milling [J]. Journal of Alloys and Compounds, 2005, 404: 712-715.
L. Hydrogen production from highly corroding Mg-based materials elaborated by ball milling [J]. Journal of Alloys and Compounds, 2005, 404: 712-715.
[21] SOLER L,  M, CASADO J. Aluminum and aluminum alloys as sources of hydrogen for fuel cell applications [J]. Journal of Power Sources, 2007, 169: 144-149.
M, CASADO J. Aluminum and aluminum alloys as sources of hydrogen for fuel cell applications [J]. Journal of Power Sources, 2007, 169: 144-149.
[22] GROSJEAN M H, ZIDOUNE M,  L, HUOT J Y. Hydrogen production via hydrolysis reaction from ball-milled Mg-based materials [J]. International Journal of Hydrogen Energy, 2006, 31: 109-119.
L, HUOT J Y. Hydrogen production via hydrolysis reaction from ball-milled Mg-based materials [J]. International Journal of Hydrogen Energy, 2006, 31: 109-119.
[23] NOVIKOV G V, KULIKOVA L N, BOGDANOVA O Y. Low-temperature chemical processes of the formation of ore manganese minerals of ferromanganese deposits of the ocean [J]. Doklady Earth Sciences, 2011, 436: 138-42.
[24] HUANG Ming-hong, OUYANG Liu-zhang, WANG Hui, LIU Jiang-wen, ZHU Min. Hydrogen generation by hydrolysis of MgH2 and enhanced kinetics performance of ammonium chloride introducing [J]. International Journal of Hydrogen Energy, 2015, 40: 6145-6150.
[25] ZHENG Jie, YANG Deng-chen, LI Wei, FU He, LI Xing-guo. Promoting H2 generation from the reaction of Mg nanoparticles and water using cations [J]. Chemical Communications, 2013, 49: 9437-9439.
氯化盐对氢化燃烧合成法及机械球磨法制备的MgH2水解性能的影响
李 姝,甘德宇,朱云峰,刘雅娜,张 戈,李李泉
南京工业大学 材料科学与工程学院,南京 210009
摘 要:研究氯化盐对氢化燃烧合成法及机械球磨法(HCS+MM)制备的镁基氢化物水解制氢动力学性能的影响。XRD分析表明HCS法可成功制备高纯MgH2。水解性能测试表明在球磨过程中添加氯化盐有利于加快水解初期反应速率及增加60 min的制氢量。MgH2-10%NH4Cl复合物具有最好的水解性能,室温下水解60 min制氢量为1311 mL/g,转化率为85.69%。这可能是因为氯化盐在球磨过程中不仅起到了球磨助剂的作用,而且在活性材料上产生了新鲜表面,促进了水解反应。
关键词:MgH2;制氢量;水解;氯化盐;氢化燃烧合成法;机械球磨
(Edited by Yun-bin HE)
Foundation item: Projects (51571112, 51171079, 51471087) supported by the National Natural Science Foundation of China; Project (13KJA430003) supported by Jiangsu Higher Education Institutions of China; Project supported by Qing Lan Project, China; Project supported by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, China
Corresponding author: Li-quan LI; Tel: +86-25-83587255; E-mail: lilq@njtech.edu.cn
DOI: 10.1016/S1003-6326(17)60062-1
Abstract: The effects of chloride salts (NaCl, MgCl2 and NH4Cl) on the hydrolysis kinetics of MgH2 prepared by hydriding combustion synthesis and mechanical milling (HCS+MM) were discussed. X-ray diffraction (XRD) analyses show that high-purity MgH2 was successfully prepared by HCS. Hydrolysis performance test results indicate that the chloride salt added during the milling process is favorable to the initial reaction rate and hydrogen generation yield within 60 min. A MgH2-10% NH4Cl composite exhibits the best performance with the hydrogen generation yield of 1311 mL/g and a conversion rate of 85.69% in 60 min at room temperature. It is suggested that the chloride salts not only play as grinding aids in the milling process, but also create fresh surface of reactive materials, favoring the hydrolysis reaction.


