DOI:10.19476/j.ysxb.1004.0609.2019.01.09
SPS法制备Ti-3Zr-2Sn-3Mo-25Nb合金的组织与性能
麻西群,于振涛,刘汉源,牛金龙,余森,贺新杰
(西北有色金属研究院 陕西省医用金属材料重点实验室,西安 710016)
摘 要:
采用等离子烧结(SPS)预合金粉末法制备了Ti-25Nb-3Zr-3Mo-2Sn(质量分数,%)(TLM)医用β型钛合金,研究TLM预合金粉末的特征和SPS制备的TLM合金在固溶时效后的组织形貌与力学性能。结果表明:等离子旋转电极法制备的TLM钛合金预合金粉末球形度好、内部致密度高,粉末凝固组织主要由细小枝晶和单晶构成。SPS制备的TLM合金其烧结态和固溶态组织为等轴β相及少量的球状α″马氏体。固溶态合金展示了良好的综合力学性能,其屈服强度约为500 MPa,抗拉强度为624 MPa,伸长率为40%,弹性模量为54.5 GPa。经500 ℃时效6 h后,合金在一定塑性的前提下,抗拉强度达到1015 MPa,弹性模量为84 GPa。
关键词:
医用β钛合金;预合金粉末;放电等离子烧结;组织形貌;力学性能;
文章编号:1004-0609(2019)-01-0074-07 中图分类号:TG146.2 文献标志码:A
医用低模量β钛合金是目前国内外热点研究的新一代无毒性和致敏性元素(Ni、V)的生物医用钛合金外科植入材料,更是未来应用潜力最大的一类生物医用钛合金[1-4],如Ti-29Nb-13Ta-4.6Zr(TNTZ)、Ti29Nb13Ta4.6Zr、 Ti-24Nb-4Zr-7.9Sn(Ti2448)、Ti-25Nb- 3Zr-3Mo-2Sn(TLM)、Ti35Nb2Ta3Zr等。这类钛合金除了具有比其他传统医用钛合金优良的生物相容性、高强度、高塑性和易于达到与人体骨弹性模量(10~30 MPa)的匹配以外,最新研究发现[5-8],它还可抑制骨萎缩促进新骨重建,以及通过弯曲等变形方法恢复或改善某些植入器械特殊部位的弹性模量,提高外科医生手术时的可操作性。TLM合金是具有我国自主知识产权的新型医用近β钛合金的一种,该材料不仅剔除了传统医用钛材中含有的Al、V等毒性或过敏性元素,其相组成非常多样化(α″、ω、α、β),不同组织下的力学特性可调范围大。此类合金这种宽泛的性能不仅能够满足目前传统外科植入用钛材的所要求性能指标,而且具有一定的可调性、超弹性等功能特性[9-10]。
但是,目前此类医用钛合金仍采用传统铸锭冶金法制备,加工过程具有污染大、成本高、批次质量不均匀等缺点。而预合金粉末冶金法制备生物医用钛合金是以钛及其合金粉末为原料,即将所需多组元成分预先分散组合,实现原子级别的均匀混合,形成多组元成分的均匀集合体,所制备的合金颗粒内部合金化充分均匀,其烧结体的合金化程度高,材料性能优越,能够避免成分偏析,得到的组织均匀、性能稳定。另一方面得到的合金晶粒细小,热加工性能也能得到很大的提高。且该法制备机械零件是一种无切削、少切削的近净成形工艺,可以大量减少机加工余量,节约金属材料,提高生产效率,降低生产成本[11-14]。由于其独特的优势,粉末冶金法制备生物医用钛合金是未来钛合金制备的一种必然趋势。而其中的放电等离子烧结技术(Spark plasma sintering,SPS)具有升降温速度快、烧结温度低、烧结时间短、致密度高等独特优点。且等离子旋转电极法制粉工艺具有很大的优势,制备过程中不需要坩埚和陶瓷喷嘴,避免了粉末制备过程的二次污染。因此,本文将采用放电等离子烧结(SPS)预合金粉末的方法制备生物医用β型钛合金TLM材料,研究 TLM预合金粉末的特征和SPS制备的TLM合金在固溶时效后的组织形貌与力学性能。
1 实验
采用等离子旋转电极(PREP)设备对近β型医用钛合金Ti-25Nb-3Zr-3Mo-2Sn铸锭进行球形预合金粉末的制备,制粉过程中电极棒高速旋转,其端面被等离子弧熔化,端面上被熔化的液滴在离心力的作用下飞出,在冷却介质(氩气)中快速凝固成细小的球形粉末颗粒。将粉末粒度小于150 μm的作为预合金粉末,其实测成分为Ti-24.5Nb-3.10Zr-2.96Mo-2.12Sn-0.095O。
采用德国FCT-HPD 25/4-SD型放电等离子烧结炉对预合金粉末进行常规烧结。将TLM粉置于烧结炉内的模具中,对模具的上下模冲施加压力,抽真空至不大于1×10-3 Pa,然后升温和SPS烧结。升温速度为100 ℃/min,烧结温度为1000 ℃,保温时间为10 min。烧结完成以后得到d52 mm×9 mm块状合金,如图1所示。对烧结制备的合金进行3种工艺的热处理,具体为固溶处理800 ℃,保温10 min后水冷(ST);固溶后380 ℃时效4 h,空冷;固溶后500 ℃时效6 h,空冷。
利用OLYMPUS金相显微镜和JSM-6700扫描电镜观察预合金粉末的组织形貌及SPS烧结块体和热处理试样的显微组织。采用SmartLab型X射线衍射仪 XRD对各组织结构进行相组成分析,采用Cu靶Kα辐射衍射,入射射线波长为λ=0.15406 nm,工作电压为40 kV,电流为30 mA,样品扫描角度范围为20°~90°,扫描速度为10 (°)/min。采用AG-Xplus 100 kN型电子万能实验机进行材料的拉伸试验,拉伸速率为0.5 mm/min。拉伸试样的尺寸如图2所示。

图1 SPS烧结制备的TLM钛合金试样
Fig. 1 TLM alloy specimens prepared by SPS method
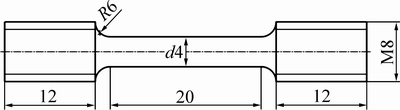
图2 拉伸试样尺寸图
Fig. 2 Schematic drawing of the specimen for tensile test (Unit: mm)
2 结果与分析
2.1 TLM预合金粉末的形貌与微观组织特征
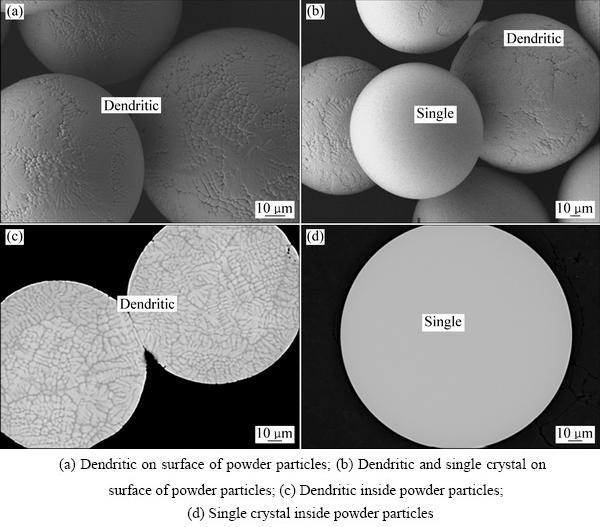
图3 TLM预合金粉末的形貌
Fig. 3 Morphologies of TLM prealloyed powder
图3所示为TLM预合金粉末颗粒的貌及粉末颗粒截面组织。由图3可见,等离子旋转电极法制备的预合金球形粉末致密度高、无孔洞结构,且未发现球形颗粒之间的焊合现象。球形粉末表面及截面呈现出树枝晶和单晶两种快速凝固组织,如图3(a)、(b)和(d)所示。图3(c)所示为枝晶的截面组织,且树枝晶的一次和二次枝晶并不发达,每个细小枝晶的直径尺寸约为3 μm,局部呈现出胞状化趋势。图3(d)所示为单晶粉末球的截面组织。与气雾化法制备的粉末相比,该法在球形度、致密度及流动性方面更佳。
2.2 TLM预合金粉末的相组成及元素偏析分析
图4所示为TLM预合金粉末的XRD谱。由图4中可看出,粉末颗粒由β相和α″相组成。图5所示为TLM合金粉末颗粒的电子探针EPMA元素线扫描图谱。图中分析了预合金粉末颗粒内部的元素偏析状态。图中显示,Nb元素集中在图中白色枝晶区域。这是因为Nb为β稳定元素,冷却时优先聚集在β相区,导致在该区域含量的升高以及Ti原子的减少。Mo、Zr、Sn、O元素则在合金中均匀分布或含量波动超过检测精度。
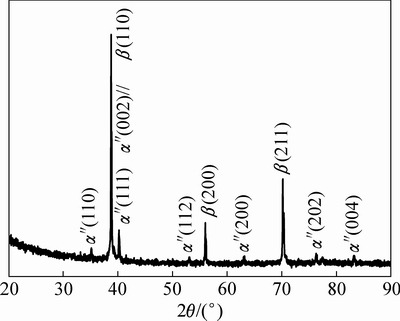
图4 TLM预合金粉末的XRD谱
Fig. 4 XRD patterns of TLM prealloyed powder
2.3 热处理对TLM SPS烧结块材的SEM微观形貌
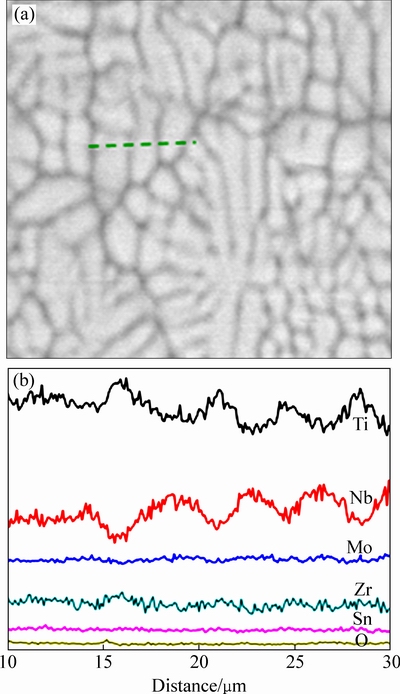
图5 预合金粉末的形貌及电子探针EPMA元素线扫描分布图
Fig. 5 Morphology (a) and EPMA analysis of elemental concentration (b) of prealloyed powder along black dotted line
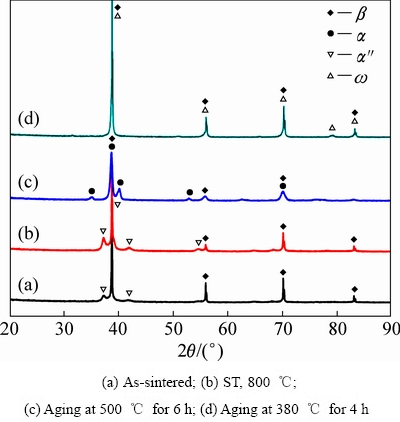
图6 SPS法制备的TLM合金不同热处理条件下的XRD谱
Fig. 6 XRD patterns of TLM alloy prepared by SPS method after different heat treatments
图6所示为SPS制备的TLM块材在不同热处理后的XRD谱。图中表明,烧结态和固溶处理后的相结构组成均为β相和α″相,这与大多数介稳或亚稳β钛合金固溶处理后的相结构一致[15-17]。但SPS法制备的Ti2448亚稳β钛合金的研究结果表明[16],烧结态时组织主要由β相和少量残留的α相组成,而固溶处理后的合金由β相、初生α相及淬火马氏体次生组成。TLM SPS烧结材料经380 ℃时效后,合金中析出了硬质的ω相,α″相均消失。当时效温度为500 ℃后,β基体中只有α相存在,ω相全部消失。由此可见,SPS制备的TLM合金的相结构演化过程与普通铸锭冶金制备的合金基本相同,都表现为β→ω相→ω相消 失→α相形成。
图7所示为SPS制备的TLM合金在不同热处理状态下的组织形貌。结合图5的分析结果显示,烧结态的显微组织为β等轴晶和细小的α″相弥散相,等轴β晶尺寸为40~100 μm之间,晶界明显,无孔洞,晶粒尺寸明显小于粉末球的平均直径,表明细小的枝晶粉末球之间形成了良好的冶金固溶,合金均匀度和致密度较好。经800 ℃固溶处理后,组织中仍以β相为主,椭球状α″相弥散分布在β相中,其中晶界处α″相的含量较多,尺寸较大,分布较密集(见图6(b))。这种现象的发生是由于晶界处的形核能较低,析出相优先在此形核,并且晶界处相的长大速度快于晶内。对固溶处理的试样进行380 ℃时效后,组织中晶界形貌清晰,晶粒内部优先形成了大量细小的弥散相,根据图6分析说明该析出相为ω相,这与常规制备方法相同工艺时效后获得的显微组织基本一致,如图7(c)所示。当时效温度升高至500 ℃后,析出相进一步长大呈短针状α相,相互交错,长度约为0.2~0.4 μm,且晶界处析出相的尺寸大于晶内,长度达到1 μm以上。这与常规熔炼获得的TLM块材的热处理结果相比,SPS制备的TLM合金在500 ℃时效析出的α相尺寸较小,密度较大,这与预合金粉末球本身具有的细小枝晶有关。
2.4 SPS法制备的TLM钛合金的力学性能
图8所示为SPS 制备的TLM钛合金在不同热处理条件下的力学性能。采用SPS制备出的TLM合金材料经过800 ℃固溶处理(ST)后,合金展示了良好的综合力学性能,其屈服强度约为500 MPa,抗拉强度为624 MPa,伸长率达到40%,弹性模量为54.5 GPa。与常规方法制备的相同规格板材力学性能相比,其在保持低弹性模量和高塑性的同时,强度提高了约100 MPa,这可能是由于析出α″马氏体的强化作用(见图7(b))。细小球状的α″马氏体相虽然在晶界处分布,但未形成连续相隔绝晶内基体的塑性变形,导致其断裂应变没有下降。这种强化相在晶界分布实现钛合金良好综合力学性能已有大量文献报道[19-20]。当合金继续进行380 ℃时效4 h后,基体中生成了大量的细小弥散分布的ω相,此时合金抗拉强度达到1000 MPa以上,伸长率约为5%,这与大量硬脆ω相的分布有关。时效温度上升至500 ℃后,弹性模量为84 GPa,由于大量密集的短针状α相的析出,合金的伸长率为8.6%,屈服强度达到980 MPa,抗拉强度达到1015 MPa,这就接近由β和ω相两种组织构成的合金强度,且这比常规棒材时效后的强度高出130 MPa以上[21]。综上所述可知,通过固溶处理后时效处理(STA)及预合金粉末法两种组织调控的方法来调节合金中不同相变的尺寸、分布、形貌等,并在获得高强度的同时保持低的弹性模量,最终达到提高合金综合力学性能的目的。
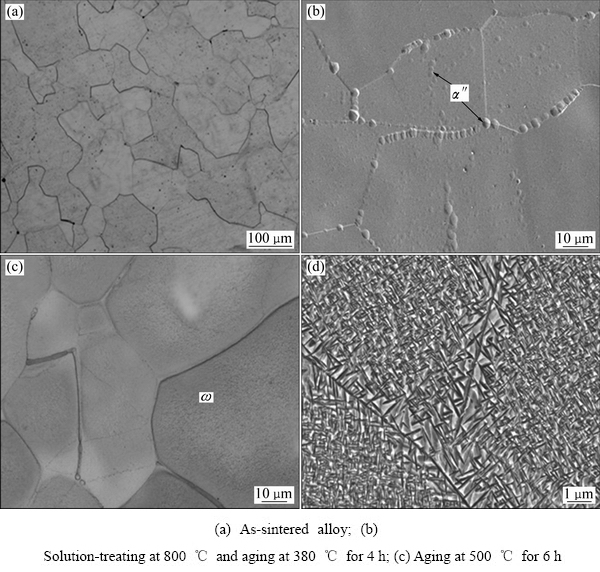
图7 SPS法制备的TLM合金不同热处理条件后的显微组织
Fig. 7 Microstructures of TLM alloy prepared by SPS method after different heat treatments
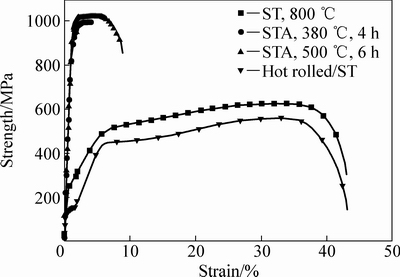
图8 SPS法制备的TLM合金在不同热处理条件下的力学性能
Fig. 8 Mechanical properties of TLM alloy prepared by SPS method after different heat treatments
3 结论
1) 等离子旋转电极法制备的生物医用β型钛合金预合金粉末球形度好,致密度高、粒度分布均匀。粉末的表面及内部形貌由细小的单晶球和树枝晶球组成,其相结构由β相和α″相构成。
2) SPS制备的TLM钛合金其烧结态及固溶态组织均以β相为主,少量球状马氏体α″优先在晶界析出。在380 ℃、500 ℃时效后的组织中,与常规方法相比,析出的ω相和相互交叉的针状α析出相更加细小、分布更加密集。
3) SPS制备的TLM钛合金经固溶处理后,合金展示了良好的综合力学性能,其屈服强度约为500 MPa,抗拉强度为624 MPa,伸长率达到40%,弹性模量为54.5 GPa。与常规方法制备的板材力学性能相比,SPS制备的TLM钛合金在保持低弹性模量和高 塑性的同时,强度提高了约100 MPa。
REFERENCES
[1] LI Yu-hua, YANG Chao, ZHAO Hai-dong, QU Sheng-guan, LI Xiao-qiang, LI Yuan-yuan. New developments of Ti-based alloys for biomedical applications[J]. Materials, 2014, 7(3): 1709-1800.
[2] NIINOMI M, BOEHLERT C J. Titanium alloys for biomedical applications[M]. Berlin Heidelberg: Springer, 2015: 179-213.
[3] 段永刚, 丁英奇, 张 龙, 刘玉章, 唐晓龙. 新型β钛合金Ti35Nb3Zr2Ta在人工关节假体应用中的生物相容性[J]. 中国组织工程研究, 2015, 19(34): 5536-5541.
DUAN Yong-gang, DING Ying-qi, ZHANG Long, LIU Yu-zhang, TANG Xiao-long. Biocompatibility of Ti35Nb3Zr2Ta a new beta-titanium alloy as joint prosthesis material[J]. Chinese Journal of Tissue Engineeing Research, 2015, 19(34): 5536-5541.
[4] 李 季, 李述军, 白 芸, 郝玉林, 杨 锐. 氟离子含量对Ti2448纳米晶在模拟唾液中腐蚀行为的影响[J]. 中国有色金属学报, 2013, 23(S1): s316-s319.
LI Ji, LI Shu-jun, BAI Yun, HAO Yu-lin, YANG Rui. Electrochemical behavior of nanostructured Ti2448 alloy in artficial saliva at 37 ℃ with fluoride[J]. The chinese Jeournal of Nonferrous Metals, 2013, 23(S1): s316-s319.
[5] BREME H Affiliated withLehrstuhl für Metallische Werkstoffe, Universitat des Saarlandes, BIEHL VAffiliated withLehrstuhl für Metallische Werkstoffe, Universitat des Saarlandes, REGER NAffiliated withDepartment of Chemistry and Biochemistry, Bayer School of Natural and Environmental Sciences, GAWALT E. Metallic biomaterials: introduction[C]. Handbook of Biomaterial Properties. New York: Springer, 2016: 151-158.
[6] YU Zhen-tao, ZHANG Ming-hua, TIAN Yu-xing, CHENG Jun, MA Xi-qun, LIU Han-yuan, WANG Chang. Designation and development of biomedical Ti alloys with finer biomechanical compatibility in long-term surgical implants[J]. Frontiers of Materials Science, 2014, 8(3): 219-229.
[7] YILMAZER H, NIINOMI M, NAKAI M, CHO K, HIEDA J. Mechanical properties of a medical β-type titanium alloy with specific microstructural evolution through high-pressure torsion[J]. Materials Science and Engineering C, 2013, 33: 2499-2507.
[8] ZHU Yong-feng, WANG Li-qiang, WANG Min-min. Superelastic and shape memory properties of TixNb3Zr2Ta alloys[J]. Journal of the Mechanical behavior of Biomedical Materials, 2012, 12(8): 151-159.
[9] 麻西群, 于振涛, 牛金龙, 余 森, 程 军. 热处理对Ti-3Zr-2Sn-3Mo-25Nb合金超弹性的影响[J]. 稀有金属材料与工程, 2016, 45(6): 1588-1592.
MA Xi-qun, YU Zhen-tao, NIU Jin-long, YU Sen, CHENG Jun. Effect of heat trreatment on superelasticity in Ti-3Zr-2Sn-3Mo-25Nbn alloy[J]. Rare Metal Materials and Engineering, 2016, 45(6): 1588-1592.
[10] 麻西群, 于振涛, 牛金龙, 余 森, 刘春潮. 超细晶TLM钛合金复合板材的组织与性能[J]. 稀有金属材料与工程, 2014, 43(9): 152-155.
MA Xi-qun, YU Zhen-tao, NIU Jin-long, YU Sen, LIU Chun-chao. Microstructure and properties of ultrafine grained TLM alloy ARB sheet[J]. Rare Metal Materials and Engineering, 2014, 43(9): 152-155.
[11] 于振涛, 田宇兴, 余 森, 麻西群. 新型骨科用β型TLM钛合金材料研究[J]. 中国骨科临床与基础研究, 2013, 5(1): 6-13.
YU Zhen-tao, TIAN Yu-xing, YU Sen, MA Xi-qun. Research on a new β type TLM titnaium alloy for orthopaedic application[J]. Chinese Journal of Clinical and Basic Research, 2013, 5(1): 6-13.
[12] HE Zheng-yuan, ZHANG Lei, SHAN Wen-rui, ZHANG Yu-qin, ZHOU Rong, JIANG Ye-hua, TAN Jun. Mechanical and corrosion properties of Ti-35Nb-7Zr-xHA composites fabricated by spark plasma sintering[J]. Transactions of Nonferrous Metals Society of China, 2017, 27(4): 848-856.
[13] 徐 伟, 路 新, 夏 青, 韩 刚, 王 涛,曲选辉. 粉末冶金超细晶医用Ti-Mo-Fe合金的制备与表征[J]. 稀有金属材料与工程, 2017, 46(5): 1393-1398.
XU Wei, LU Xin, XIA Qing, HAN Gang, WANG Tao, QU Xuan-hui. Fabrication and characterization of PM ultrafine grained Ti-Mo-Fe alloys for biomedical application[J]. Rare Metal Materials and Engineering, 2017, 46(5): 1393-1398.
[14] BISWAS K, HE J, BLUM I D WU C L, HOGAN T P. High performance bulk thermoelectrics with all-scale hierarchical architectures[J]. Nature, 2012, 489(7416): 414-418.
[15] LI Q, NIINOMI M, HIEDA J, NAKAI M, CHO K. Deformation induced ω phase in modified Ti-29Nb-13Ta-4.6Zr alloy by Cr addition[J]. Acta Biomaterials, 2013, 9(8): 8027-8035.
[16] NIINOMI M, NAKAI M, HIEDA J. Development of new metallic alloys for biomedical applications[J]. Acta Biomaterials, 2012, 8: 3888-3901.
[17] ZHAO X F, NIINOMI M, NAKAI M, HIEDA J, ISHIMOTO T. Optimization of Cr content of metastable β-type Ti-Cr alloys with changeable Young’s modulus for spinal fixation applications[J]. Acta Biomaterials, 2012, 8(6): 2392-2400.
[18] 刘万理, 张玉勤, 蒋业华, 周 荣. 固溶温度对SPS烧结Ti-24Nb-4Zr-8Sn合金组织和力学性能的影响[J]. 金属热处理, 2017, 42(4): 99-103.
LIU Wan-li, ZHANG Yu-qin, JIANG Ye-hua, ZHOU Rong. Effect of solution temperature on microstructure and mechanical properties of Ti-24Nb-4Zr-8Sn alloy prepared by spark plasma sintering[J]. Heat Treatment of Metals, 2017, 42(4): 99-103.
[19] ZHANG Yu-sheng, ZHAO Yong-hao, ZHANG Wei, LU Jin-wen, HU Jiang-jiang, HUO Wang-tu, ZHANG Ping-xiang. Core-shell structured titanium-nitrogen alloys with high strength, high thermal stability and good plasticity[J]. Scientific Reports, 2017, 7: 1-7.
[20] ZHANG Yu-sheng, WANG Xin, ZHANG Wei, HUO Wang-tu, HU Jiang-jiang. Elevated tensile properties of Ti-O alloy with a novel core-shell structure[J]. Materials Science and Engineering A, 2017, 696: 360-365.
[21] 麻西群, 憨 勇, 于振涛, 孙巧艳, 牛金龙, 袁思波. 骨科植入用TLM钛合金的相转变与力学性能[J]. 稀有金属材料与工程, 2012, 41(9): 1535-1538.
MA Xi-qun, HAN Yong, YU Zhen-tao, SUN Qiao-yan, NIU Jin-long, YUAN Si-bo. Phase transformation and mechanical properties of TLM titanium alloy for orthopaedic implant application[J]. Rare Metal Materials and Engineering, 2012, 41(9): 1535-1538.
Microstructure and properties of Ti-3Zr-2Sn-3Mo-25Nb alloy prepared by SPS method
MA Xi-qun, YU Zhen-tao, LIU Han-yuan, NIU Jin-long, YU Sen, HE Xin-jie
(Shaanxi Key Laboratory of Biomedical Metal Materials, Northwest Institute for Nonferrous Metal Research, Xi’an 710016, China)
Abstract: A biomedical β-Ti alloy Ti-25Nb-3Zr-3Mo-2Sn (mass fraction, %) was fabricated by spark plasma sintering (SPS). The characteristics, morphology and mechanical properties of TLM prealloyed powder after solid solution and aging were investigated. The results show that TLM prealloyed power prepared by plasma rotating electrode process has good spherical shape and high internal density. The solidification microstructure is consisted by small dendritic and single crystal. The sintered and as-soluted microstructures of TLM alloy propared by SPS method are equiaxed β phase and α″ martensite. The as-soluted alloy exhibits good comprehensive mechanical preperties with yield strength of 500 MPa, tensile strength of 624 MPa, elongation of 40% and elastic modulus of 54.5 GPa. Ageing at 500 ℃ for 6 h, after a superior the alloy has good plasticity, tensile strength reaches up to 1015 MPa, and the elastic modulus is 84 GPa.
Key words: medical β titanium alloy; prealloyed powder; spark plasma sintering; microstructure; mechanical property
Foundation item: Project(2016KTCG01-04) supported by Transformation Program of Major Scientific and Technological Achievements of Shaanxi Province, China; Project(31400821) supported by National Natural Science Foundation of China; Project(2015GY160) supported by Industrial Science and Technology Program of Shaanxi Province, China
Received date: 2017-11-03; Accepted date: 2018-04-10
Corresponding author: YU Zhen-tao; Tel: +86-29-86222297; E-mail: maxiqun23@126.com
(编辑 李艳红)
基金项目:陕西省重大科技成果转化项目(2016KTCG01-04);国家自然科学基金资助项目(31400821);陕西省工业科技攻关项目(2015GY160)
收稿日期:2017-11-03;修订日期:2018-04-10
通信作者:于振涛,教授,博士;电话:029-86222297;E-mail:maxiqun23@126.com
摘 要:采用等离子烧结(SPS)预合金粉末法制备了Ti-25Nb-3Zr-3Mo-2Sn(质量分数,%)(TLM)医用β型钛合金,研究TLM预合金粉末的特征和SPS制备的TLM合金在固溶时效后的组织形貌与力学性能。结果表明:等离子旋转电极法制备的TLM钛合金预合金粉末球形度好、内部致密度高,粉末凝固组织主要由细小枝晶和单晶构成。SPS制备的TLM合金其烧结态和固溶态组织为等轴β相及少量的球状α″马氏体。固溶态合金展示了良好的综合力学性能,其屈服强度约为500 MPa,抗拉强度为624 MPa,伸长率为40%,弹性模量为54.5 GPa。经500 ℃时效6 h后,合金在一定塑性的前提下,抗拉强度达到1015 MPa,弹性模量为84 GPa。
[4] 李 季, 李述军, 白 芸, 郝玉林, 杨 锐. 氟离子含量对Ti2448纳米晶在模拟唾液中腐蚀行为的影响[J]. 中国有色金属学报, 2013, 23(S1): s316-s319.
[9] 麻西群, 于振涛, 牛金龙, 余 森, 程 军. 热处理对Ti-3Zr-2Sn-3Mo-25Nb合金超弹性的影响[J]. 稀有金属材料与工程, 2016, 45(6): 1588-1592.
[10] 麻西群, 于振涛, 牛金龙, 余 森, 刘春潮. 超细晶TLM钛合金复合板材的组织与性能[J]. 稀有金属材料与工程, 2014, 43(9): 152-155.
[11] 于振涛, 田宇兴, 余 森, 麻西群. 新型骨科用β型TLM钛合金材料研究[J]. 中国骨科临床与基础研究, 2013, 5(1): 6-13.
[13] 徐 伟, 路 新, 夏 青, 韩 刚, 王 涛,曲选辉. 粉末冶金超细晶医用Ti-Mo-Fe合金的制备与表征[J]. 稀有金属材料与工程, 2017, 46(5): 1393-1398.
[18] 刘万理, 张玉勤, 蒋业华, 周 荣. 固溶温度对SPS烧结Ti-24Nb-4Zr-8Sn合金组织和力学性能的影响[J]. 金属热处理, 2017, 42(4): 99-103.
[21] 麻西群, 憨 勇, 于振涛, 孙巧艳, 牛金龙, 袁思波. 骨科植入用TLM钛合金的相转变与力学性能[J]. 稀有金属材料与工程, 2012, 41(9): 1535-1538.


