
Effect of addition of Zn and Al elements on glass-forming ability and thermal stability of Mg-Cu-Y bulk metallic glasses
LI Ye-sheng(黎业生), FU Qun-qiang(付群强), WU Zi-ping(吴子平), DONG Ding-qian (董定乾)
(School of Materials and Chemical Engineering, Jiangxi University of Science and Technology, Ganzhou 341000, China)
Received 15 July 2007; accepted 10 September 2007
Abstract:
After substituting partial Cu and Mg with Zn or Al elements for Mg65Cu25Y10 alloy, respectively, the metallic glass plate samples with thickness of 2-3 mm were prepared by water-quenching, their respective glass-forming ability and thermal stability were studied by using differential thermal analysis (DTA) and X-ray diffraction (XRD). Using Kissinger equation, the activation energies of crystallization of these metallic glasses heated with a constant rate were calculated. The results show that Al element is greatly harmful to the glass-forming ability of Mg-Cu-Y alloys and cannot acquire bulk amorphous alloys; nevertheless, the effect of Zn element addition is indeterminate for various components. The magnitudes of thermal stability are also revealed.
Key words:
Mg-based alloy; metallic glasses; glass-forming ability; thermal stability;
1 Introduction
Mg-based alloys were paid more and more attention for their low density and high specific strength, specific stiffness in recent years. Mg-based metallic glasses are considered structure materials due to their high specific strength and other attractive mechanical and corrosion resistance properties. Since the glass-forming ability of Mg-based bulk metallic glass was developed greatly in the end of last century, the alloy systems and preparation methods were improved[1-5]. The proper addition of other elements affects the glass-forming ability and thermal stability of Mg-Cu-Y alloys[3-4]. As it is known to all, the nanocrystalline phase can be obtained from amorphous material by an appropriate heat treatment that leads to crystallization[6-7], so the control of this process is very important.
In this study, Mg65Cu20Y10Zn5, Mg60Cu25Y10Zn5 and Mg65Cu20Y10Al5 alloys were prepared for the substitution of partial Cu and Mg elements in Mg65Cu25Y10 with Zn or Al. To be compared with Mg65Cu25Y10 alloy, their glass-forming ability, thermal stability and effects of Zn and Al elements were investigated.
2 ExperimentalConsulting the nominal compositions of Mg-Cu-Y metallic glasses, four types of pure metal of Mg, Cu, Y and Zn or Al were mixed and melted in the medium frequency-induced vacuum furnace to obtain the parent alloys with design composition. The parent alloys were smashed and milled into powders, and then the powders were put into a copper mould with a cavity of d 20 mm×5 mm and sealed. The copper mould was quickly heated to 700 ℃ in the above vacuum furnace, holding for 1 min, then quenched into the mixture of ice and water, and a plate sample with the thickness of 2-3mm was obtained.
The structure of the plate sample was determined by Miniflex XRD instrument, and its behavior of glass transition, crystallization and melting were detected by Diamond TG/DTA 6000 thermal analyzer, using α-Al2O3 as reference and protected by flow Ar gas with high purity. The chemical composition of parent alloys was measured by Magix(PW-2424) type of X fluorescence analyzer.
3 Results and discussion
3.1 Glass-forming ability of the alloys
Table 1 shows the chemical compositions of the parent alloys.
Table 1 Chemical compositions of Mg-Cu-Y-Zn and Mg-Cu-Y-Al parent alloys (mass fraction, %)

Changing mass fraction into mole fraction in Table 1, alloy A is Mg65Cu20Y10Zn5, alloy B is Mg59Cu26Y10Zn5 and alloy C is Mg64.1Cu20.9Y9.5Al5.4; apparently, the chemical compositions of parent alloys are consistent with the nominal compositions.
Fig.1 shows the XRD patterns and DTA curves of water-quenching samples of parent alloys, respectively. Mg65Cu20Y10Zn5 and Mg59Cu26Y10Zn5 quenched samples only exhibit a broad peak, indicating the formation of single amorphous phase. However, Mg64.1Cu20.9Y9.5Al5.4 quenched sample shows several sharp crystalline peaks, indicating the coexistence of Mg2Cu, Al2CuMg, YAl3, and little unknown phase, as shown in Fig.1(a). The DTA curves of the above three type samples all exhibit clear exothermal peaks, as shown in Fig.1(b). From Fig.1, it can be deduced that the water-quenching samples of Mg65Cu20Y10Zn5 and Mg59Cu26Y10Zn5 form amorphous phase; as the heating temperature increases, they will be crystallized. The water-quenching sample of Mg64.1Cu20.9Y9.5Al5.4 almost cannot form amorphous phase, but it forms an unstable phase; as the heating temperature increases, the unstable phase will transform into a stable one. The above evidences demonstrate that the substitution of partial Cu for Al is harmful to Mg65Cu25Y10 alloy, which decreases the glass-forming ability of Mg65Cu25Y10 alloy greatly.
Table 2 lists the data of the glass transition temperature (Tg), onset temperature of crystallization (Tx1), supercooled liquid region (?Tx), reduced glass transition temperature (Trg) and melting temperature(Tm) of Mg65Cu25Y10, Mg60Cu30Y10, Mg65Cu20Y10 Zn5 and Mg59Cu26Y10Zn5 metallic glasses with a heating rate of 20 ℃/min.
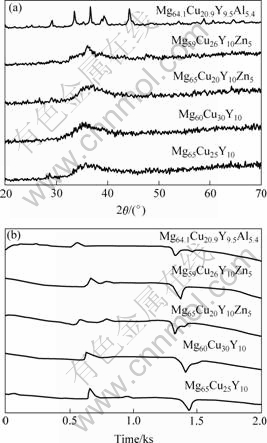
Fig.1 XRD patterns (a) and DTA curves (b) of water quenched alloys
Table 2 Data of Tg , Tx1, ?Tx , Trg and Tm obtained with DTA for metallic glasses with heating rate of 20 ℃/min
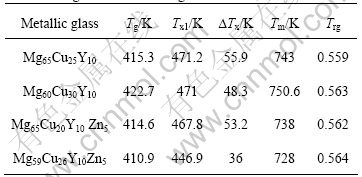
Mg65Cu20Y10 Zn5 metallic glass possesses lower Tg by little, Tx1 by about 3.4 K and Tm by about 5 K than Mg65Cu25Y10, and its DTA trace has a trend of moving forward, compared with Mg65Cu25Y10. Mg60Cu25Y10 Zn5 metallic glass has lower Tg by 11.8 K, Tx1 by 24.1 K and Tm by 22.6 K than Mg60Cu30Y10. Compared with the thermal parameters of Mg65Cu20Y10Zn5, the thermal parameters of Mg59Cu26Y10Zn5 have larger changes and its DTA trace moves forward more prominently. From the view of glass-forming, the supercooled liquid region ?Tx of Mg65Cu25Y10 decreases about 2.7 K after replacing Cu with Zn, whereas the reduced glass transition temperature Trg increases slightly, from 0.559 to 0.562; ?Tx of Mg60Cu30Y10 decreases about 12.3 K after replacing Cu with Zn, and Trg keeps at 0.563, these similar phenomena also appear in Zr-Ti-Cu-Ni-Be and Cu-Zr-Ti alloys[8-11]. From the preparation process of the sample, it can be seen that the decrease of ?Tx does not exhibit the descendant of the glass forming ability. It demonstrates that the glass forming ability is not only determined by ?Tx, Trg is also an important reference index. Comparing Mg65Cu20Y10Zn5 with Mg60Cu25Y10- Zn5, the former has a larger ?Tx about 17.2 K than the latter, both almost have the same value of Trg, which shows that the former has a strong glass forming ability than the latter, as the same as the situation of metallic glasses without Zn.
3.2 Thermal stability
In order to study the crystallization processes of Mg65Cu20Y10Zn5 and Mg59Cu26Y10Zn5 metallic glasses, the DTA traces were measured at various heating temperature rates of 10, 20, 30, 40 and 50 K/min, respectively. Both of the DTA traces with a constant heating rate of 20 K/min of Mg65Cu20Y10Zn5 and Mg59Cu26Y10Zn5 metallic glasses show two exothermal peaks, i.e. the crystallization process divides two stages. The first peak of Mg65Cu20Y10Zn5 is big, and the second is small; whereas the DTA trace of Mg59Cu26Y10Zn5 shows two big exothermal peaks, as shown in Fig.1(b). Fig.1(b) only shows the DTA curves with a constant heating rate of 20 K/min, similar DTA curves are also obtained for other heating rates. This illustrates that the crystallization of amorphous Mg65Cu20Y10Zn5 mainly focuses on the first stage; the crystallization amount of the second one is less relatively. Contrarily, the crystallization amounts of the two stages are big for Mg59Cu26Y10Zn5. As heating rate increases, both Tx1 and Tp of all metallic glasses increase, and the difference ?Tpx (?Tpx = Tp-Tx1) between Tp and Tx1 also increases, as listed in Tables 3-6.
Table 3 Initial and peak temperatures of crystallization for Mg65Cu25Y10 metallic glass with various heating rates
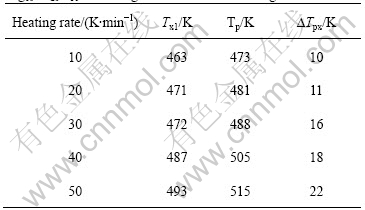
Table 4 Initial and peak temperatures of crystallization for Mg60Cu30Y10 metallic glass with various heating rates
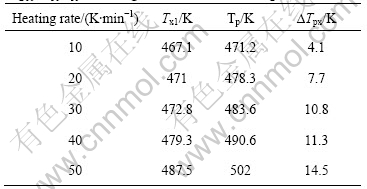
Table 5 Initial and peak temperatures of crystallization for Mg65Cu20Y10Zn5 metallic glass with various heating rates
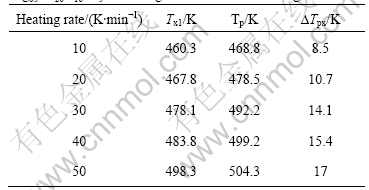
Table 6 Initial and peak temperatures of crystallization for Mg59Cu26Y10Zn5 metallic glass with various heating rates

Using Kissinger equation, ln(r/T2)=-E/RT+constant, and the data of Tables 3-6, the lines can be obtained from the diagrams of ln(T2/r) vs 1/T, as shown in Fig.2. The slope of line is E/R, and the activity energy of crystallization, E, under different conditions can be calculated, whose values are as follows.
Put Tx1 into Kissinger equation, the activity energies of crystallization of Mg65Cu25Y10, Mg60Cu30Y10, Mg65Cu20Y10Zn5 and Mg59Cu26Y10Zn5 metallic glasses are 78.66, 127.97, 96.47 and 81.78 kJ/mol, respectively. Put Tp into Kissinger equation, E values of them are 56.92, 92.32, 78.92 and 66.27 kJ/mol, respectively.
From the above results, it can be seen that E values of amorphous Mg65Cu20Y10Zn5 and Mg59Cu26Y10Zn5 are similar to those of Mg65Cu25Y10 and Mg60Cu30Y10, as putting Tx1 and Tp into Kissinger equation, E values of the former are larger than those of the latter. The reason is that while Tp>Tx1, atoms diffuse faster for possessing

Fig.2 Kissinger plots of DTA of water-quenching metallic glasses: (a) Mg65Cu25Y10; (b) Mg60Cu30Y10; (c) Mg65Cu20Y10Zn5; (d) Mg59Cu26Y10Zn5
higher energy and bigger momentum at high temperature; there exists partial crystallization in the front of crystallization process, the potential energy that atoms need to get across is lower. So the E values calculated from Tx1 is higher. Putting either Tx1 or Tp into Kissinger equation, the E value of amorphous Mg65Cu20Y10Zn5 is larger than that of Mg65Cu25Y10, demonstrating that the thermal stability of Mg65Cu25Y10 substituting Cu with Zn gets stronger from the results of crystallization with a constant heating rate, according with that of isothermal annealing. E value of amorphous Mg59Cu26Y10Zn5 is lower than that of amorphous Mg60Cu30Y10, demonstrating that the thermal stability of Mg60Cu30Y10 substituting Cu with Zn gets weaker. E value of amorphous Mg65Cu20Y10Zn5 is bigger than that of amorphous Mg59Cu26Y10Zn5, demonstrating that the former possesses the thermal stability higher than the latter does.
4 Conclusions1) Mg65Cu25Y10 alloy substituting partial Cu with Al can’t obtain amorphous structure by water-quenching; the addition of Al element is very harmful to the glass-forming ability of Mg65Cu25Y10 alloy.
2) After substituting Cu with Zn to form Mg65Cu20- Y10Zn5, ?Tx of Mg65Cu25Y10 metallic glass lowers slightly, whereas Trg lifts a little, glass-forming ability doesn’t weaken distinctly, and thermal stability improves remarkably, the thermal stability temperature rises from 423 K to 473 K.
3) The glass-forming ability of Mg60Cu30Y10 doesn’t decrease obviously, but whose thermal stability decreases from 533 K to 423 K while substituting Cu with Zn to form Mg59Cu26Y10Zn5. The thermal stability of Mg65 Cu20Y10Zn5 is 50 K higher than that of Mg59 Cu26Y10Zn5 during the process of isothermal crystallization.
4) With the comparison of glass-forming abilities of Mg65 Cu20Y10Zn5 and Mg59Cu26Y10Zn5, the former has larger ?Tx and Trg than the latter does, which shows that Mg65Cu20Y10Zn5 alloy has stronger glass-forming ability than Mg59Cu26Y10Zn5, as the same as those without Zn.
AcknowledgementsThe authors gratefully acknowledge the financial supports from the Science and Technology Foundation of Education Department and Industrial Plan of Science and Technology Bureau of Jiangxi Province, P.R.China under the contract of No.18, 2004 and No.188, 2006, respectively.
References[1] MEN H,YANG M C, XU Jian. Glass-forming ability of Mg-Cu-Co-Y alloy[J]. Mater Sci Forum, 2002, 386: 39-46.
[2] BARTUSCH B, SCHURACK F, ECKERT J. High strength magnesium-based glass matrix composites[J]. Mater Trans JIM, 2002, 43: 1979-1984.
[3] MEN H, KIM W T, KIM D H. Glass formation and crystallization behavior in Mg65Cu25Y10-xGdx (x=0, 5 and 10) alloys[J]. Journal of Non-Crystalline Solids, 2004, 337(1): 29-35.
[4] MA H, SHI L L, XU J, LI Y, MA E. Discovering inch-diameter metallic glasses in three-dimensional composition space[J]. Applied Physics Letters, 2005, 87: 181915-1-181915-3.
[5] INOUE A, KOHINATA M, OHTERA K, TSAI A P, MASUMOTO T. Mg-Ni-La amorphous alloys with a wide supercooled liquid region[J]. Mater Trans JIM, 1989, 30(5): 378-381.
[6] FECHT H J, HELLSTERN E, JOHNSON W L. Nanocrystalline metals prepared by high-energy ball milling[J]. Metallurgical and Materials Transactions A,1990, 21A: 2333-2337.
[7] LU K, WANG J T, WEI W D. A new method for synthesizing nanocrystalline alloys[J]. Journal of Applied Physics, 1991, 69: 522-5225.
[8] KATO H, YUBUTA K, LOUZGUINE D V, INOUE A, KIM H S. Influence of nano-precipitation on strength of Cu60Zr30Ti10 glass containing um-ZC particle reinforcements[J]. Scripta Materialia, 2004, 51(6): 577-581.
[9] PEKER A, JOHNSON W L. A highly processable metallic glass Zr41.2 Ti13.8 Cu12.5Ni10Be22.5[J]. Appl Phys, 1993, 63: 2342-2347.
[10] WU Wen-fei, YAO Ke-fu, ZHAO Zhan-kui. Influence of pulsing current on the glass transition and crystallizing kinetics of a Zr base bulk amorphous alloy[J].Chinese Science Bulletin, 2004, 49(24): 2581-2585.
[11] LOUZGUINE D V, INOUE A. Evaluation of the thermal stability of a Cu60Hf25Ti15 metallic glass[J]. Appl Phys Lett, 2002, 81(14): 2561-2562.
Corresponding author: LI Ye-sheng; Tel: +86-797-8313334; E-mail: nfyyliyesheng@tom.com


