J. Cent. South Univ. Technol. (2011) 18: 1857-1864
DOI: 10.1007/s11771-011-0914-0![]()
A strategy to produce monoclonal antibodies against gp96 by
prime-boost regimen using endogenous protein and
E. coli heterologously-expressed fragment
ZHANG Yu-dan(张誉丹)1, 2, CAO Sheng(操胜)1, MENG Song-dong(孟颂东)1, GAO George Fu(高福)1, 2, 3
1. CAS Key Laboratory of Pathogenic Microbiology and Immunology, Institute of Microbiology,Chinese Academy of Sciences, Beijing 100101, China;
2. Graduate University, Chinese Academy of Sciences, Beijing 100049, China;
3. Research Network of Immunity and Health (rNIH), Beijing Institutes of Life Science, Beijing 100101, China
? Central South University Press and Springer-Verlag Berlin Heidelberg 2011
Abstract:
Gp96, a member of HSP90 family, is a versatile molecular chaperone with various newly-discovered functions, for example to serve as a low affinity, high capacity calcium binding protein, a natural adjuvant for therapeutic cancer vaccines, a tumor rejection antigen, an immune regulator to pathological cell death. Its multi-functional and structural characteristics make it also an interesting target to develop antibody-based therapeutics. However, its low immunogenicity to mice, because of its high-sequence similarity among different species, is an obstacle to obtain valuable monoclonal antibodies (MAbs). This is a common problem for any low immunogenic proteins, whose sequences share close identity between mice and other species. Here, a new strategy of priming was employed by swine endogenous full-length gp96 and then boosting by E. coli-system heterologously expressed gp96 N-terminal fragment (N-355) to generate MAbs. Twelve different highly-specific MAbs against swine/human endogenous gp96 were successfully obtained. The binding activities of these MAbs were confirmed by enzyme-linked immunosorbent assay (ELISA), Western blot (WB), immunofluorescence and flow cytometry analysis. This provides some important reagents for further research and potential therapeutics. The methods employed can be used for MAb production of any sequence-highly-conserved proteins between mice and swine/human (or any other species).
Key words:
monoclonal antibody; priming-boost; gp96; low immunogenic protein;
1 Introduction
Heat shock protein (HSP) glycoprotein 96 (gp96), also known as glucose-regulated protein grp94, is the endoplasm reticulum (ER) counterpart of HSP90 [1-2]. Being a member of the HSP90s family, it primarily functions as a molecular chaperone which is associated with newly synthesized polypeptide chains or misfolded proteins to help them to get the correct conformation [3-8]. Thus, the expression of this protein is up-regulated by conditions, promoting protein unfolding, misfolding or aggregation, such as heat shock and virus infection, which is consistent with its primary role [9-10]. In addition to its function in protein folding and assembly, gp96 has also been known for its variety of multi-functions, such as a low-affinity, high-capacity calcium binding protein, a potential anti-tumor vaccine, and a natural adjuvant for therapeutic cancer vaccines [11-18]. Moreover, its role in ischemia and peptide binding, as well as its induction by glucose starvation, interferons and estrogen are attractive aspects which have drawn intensive investigation [12].
Previously, SRIVASTAVA’s group and other laboratories have identified gp96 as a tumor rejection antigen that is capable of inducing tumor-specific cytotoxic T-cell (CTL) responses against the tumors from which it is isolated [13-14]. This is a spectacular fact because many other peptide-binding proteins do not elicit such protective immunity. One reason for this phenomenon is the association of gp96 with antigenic peptides derived from cellular proteins or infectious pathogens [15]. Gp96 binds and transfers antigenic peptides to the major histocompatability complex (MHC) class I molecules on the surface of dendritic cells (DCs) or other antigen-presenting cells (APCs) [15], for presentation to lymphocytes (e.g., CD8 or CD4 T cells), hence triggers immune responses. In this way, it is also able to mediate the maturation and activation of DCs which are required to enable highly sophisticated APCs to prime CTL responses [16].
As mentioned above, gp96 then is considered to be an ideal anti-tumor vaccine that stimulates cellular immune response including mucosal immunity [11], because of its capacity to help the immune system to discriminate between self and nonself, or between self and altered-self [15]. In addition, it is especially suitable to alert the immune cells of danger because it is intracellular and one of the most abundant proteins of a cell [11]. All of the characteristics of gp96 together make it a highly significant molecule for both innate and adaptive immunity [12].
Monoclonal antibodies specific for gp96 are important to investigate the functions of gp96. However, the structure of gp96 is highly conserved amongst all mammalian species. Its sequence also shows high identity with HSP90. Because of the high homology of gp96 among species, as well as its low immunogenicity, only those antibodies with low affinities can be produced by the traditional immunization approach, e.g. the current commercially-available monoclonal antibodies which are not ideal as research agents. Our work described here has provided a novel way to generate MAbs in mice with high titers, high affinities and specificities, which are all determined through enzyme-linked immunosorbent assay, Western blot, immunofluorescence and flow cytometry analysis. This immunization regime of endogenous protein priming followed by boosting by heterologously-expressed protein fragment may provide a novel strategy for MAb production of highly-sequence-conserved proteins between mice and other mammalians, including humans.
2 Materials and methods
2.1 Purification of swine gp96 and preparation of homo-N-355 fragment
Gp96 protein was purified from healthy swine livers as previously described [17-19].
Briefly, 70 g swine livers were homogenized before being precipitated with 70% ammonium sulfate. The precipitate which contained gp96 protein was collected and solubilized. ConA Sepharose column (Amersham Pharmacia) was used to pool the protein afterwards, and it was followed by Source 15Q column (4.6-100 mm, GE, USA) to further purify the eluted material on an FPLC system (GE, USA). A Superdex-200 gel filtration column (GE, USA) was used as the final purification step to obtain gp96 protein. The purity of the protein was tested by Coomassie blue staining to be at least 90%. The quantity of gp96 was confirmed by bicinchoninic acid protein assay (Pierce Biotechnology, Thermo Scientific, USA).
N-355 fragment (22-376 aa) was expressed and purified as GST fusion protein in E. coli [20]. Possible contaminated endotoxin in all N-355 samples was eliminated using the procedures by WARGER et al [21] and LIU et al [22], followed by detoxi-gel endotoxin removing gel (Pierce Biotechnology, Thermo Scientific, USA). The concentration of endotoxin was examined by Limulus Amebocyte Lysate assay (BioWhittaker, Lonza, Switzerland). Its final value in N-355 purification was decreased to below 1 endotoxin unit (EU) per milligram of protein.
All buffers applied during the gp96 and N-355 fragment preparation process were made in pyrogen-free water.
2.2 Generation and purification of MAbs
The BALB/c mice were given primarily intraperitoneal injections of gp96 protein (100 μg/mouse), mixed with complete Freud’s adjuvant (Sigma-Aldrich). After two weeks of the initial immunization, the mice were boosted subsequently twice with 50 μg gp96 mixed with incomplete adjuvant (Sigma-Aldrich) at two-week interval. One week after the last injection, blood samples were taken to determine the antibody titer using ELISA. Because of the low titer, seven further injections of antigens alone were given (four boosts of full-length gp96, followed by three boosts of N-355 fragment). Three days after the last immunization, ELISA was applied again to assess the titer. The mouse of the highest titer was chosen to harvest spleen cells, which were subsequently fused with SP2/0 myeloma cells by standard procedures. The hybridoma cells were obtained and cloned by limiting dilution method. In order to ensure the monoclonality and stability of the cell lines that produced specific antibodies, subcloning was carried out by limiting dilution for 3-5 times, until the ELISA results were all positive. Twelve cell lines that secreted high-titer gp96-specific antibodies were used to generate ascites in BALB/c mice. MAbs were purified by protein G chromatography according to manufacturer’s protocol (Amersham Biosciences, GE, USA), and their concentrations were determined by bicinchoninic acid protein assay (Pierce Biotechnology, Thermo Scientific, USA).
2.3 ELISA assay (carried out with N-355 fragment and full-length gp96)
To evaluate the antigen-binding activity of the purified MAbs, ELISAs were carried out in 96-well ELISA plates. Antigens were applied to wells on an ELISA plate. The plate was incubated at 4 °C overnight; rinsed with phosphate buffered saline (PBS) containing 0.1% Tween-20 for three times and blocked with 5% skimmed milk in PBS for 1 h at 37 °C. After three times of washing, dilutions of MAbs were added to the coated wells and incubated at 37 °C for 1 h (100 μL/well). The solutions were disposed and the wells were washed three times. Diluted HRP-conjugated anti-mouse IgG was then added and incubated for 0.5-1 h at 37 °C. The wells were rinsed three times again. The reaction was stopped by adding 0.05 mL of 2 mol/L H2SO4. Absorbance at A450nm was measured with a microplate reader (BioTek, USA). A commercial monoclonal antibody of gp96 was used as positive control (Santa Cruz Biotechnology, USA), an irrelevant mice antibody (IgG) was used as negative control, and PBS was used as mock control.
2.4 Western blotting
Purified gp96 was boiled in a reducing buffer and subjected to 10% SDS-PAGE. After this, denatured protein was electro-blotted onto nitrocellulose membranes (Amersham Biosciences, GE, USA) and blocked in blocking buffer (0.05% Tween-20 in Tris-buffered saline (TBST) with 5% non-fat dry milk powder) for overnight at 4 °C. Then, the blocking solution was removed, and the membranes were incubated for 2 h at room temperature with 12 undiluted hybridoma supernatants respectively. After four times of washing in TBST, the membranes were incubated with horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (1:5 000 dilution; Jackson ImmunoResearch, USA) for 1.5 h at room temperature with shaking, and then washed four times for 10 min per wash with TBST at room temperature. SuperSignal West Pico Chemiluminescent substrate solution (Pierce Biotechnology, GE, USA) was used to detect specific antibody binding signals. A polyclonal antibody of gp96 was used as positive control (1:2000 dilution, Santa Cruz Biotechnology, USA), and SP2/0 supernatant was used as negative control.
2.5 Immunofluorescence analysis
HepG2 cells that are known for abundant expression of homo-gp96 [23-24] were grown in a 24-well plate and fixed for 15 min with 4% formaldehyde in PBS. After fixation, cells were washed three times in PBS for 5 min each, then covered with ice-cold 100% methanol to a depth of 3-5 mm and incubated for 10 min in freezer. After that, cells were washed three times in PBS as previously described, and blocked in 5% normal goat serum in PBS/0.3% Triton X-100 for 60 min at 37 °C. Cells were incubated with MAbs which were diluted in PBS/0.3% Triton X-100 with 1% bovine serum albumin (BSA) for 1 h at room temperature. After three times of washing in PBS, cells were treated to 1 h incubation with diluted Rhodamine-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, USA) at room temperature in dark, and thereafter washed in PBS for three times. Cells were stained with DAPI staining solution (1 μg/mL; ENZO (Alexis-biomol), USA) for 10 min at room temperature, followed by three times of washing in PBS. Cells without addition of primary antibody were set as control. Observation was made under fluorescence microscope.
2.6 Flow cytometry using HepG2 cell
Flow cytometry analysis of MAbs was also performed with HepG2 cells. Briefly, cells were collected and resuspended in PBS. Formaldehyde was then added to a final content of 4%, and cells were first fixed for 10 min at 37°C and then chilled on ice for 1 min. Cells were permeabilized by adding ice-cold 100% methanol slowly, while gently vortexing, to a final content of 90% methanol. After incubation on ice for 30 min, the amount of cells was counted using a hemacytometer. Around 1×106 cells were aliquoted into each assay tube, rinsed twice by centrifugation with 2-3 mL incubation buffer (1 g BSA in 200 mL PBS) and blocked in incubation buffer for 10 min at room temperature. Cells were rinsed by PBS, and then incubated with 1 mL PBS/0.3%Triton X-100 for 10 min at room temperature. After rinsing cells as described above, diluted MAbs were added into each tube (100 μg/mL) and incubated for 1 h at room temperature. Cells were rinsed again, and diluted (1:80) FITC- conjugated goat anti-mouse IgG (eBioscience, USA) was added and incubated for 30 min at room temperature. Cells were then washed again, and resuspended in 0.5 mL PBS. A FACSCalibur flow cytometer (BD Biosciences, USA) was used for analysis. Control was cells without addition of primary MAb.
3 Results
3.1 Production and purification of MAbs against gp96
Gp96 was extracted from swine liver with at least 90% purity. Because of its low immunogenicity, nothing was produced after boosting the mice by full-length gp96 with or without adjuvant. However, when E. coli expressed N-terminal fragment of the antigen protein was used to boost the gp96-immunized mice, high-titered antibody response in the immunized BALB/c mice was detected after three times of boosts. Splenocytes from the immunized BALB/c mice were fused with murine myeloma cells to generate hybridomas. The positive-fused cells were screened using ELISA. Twelve stable cell lines were finally isolated and cloned. Ascites were obtained in BALB/c mice by these hybridomas. MAbs were purified by protein G chromatography. The concentrations of the purified MAbs varied from 1 mg/mL to 17 mg/mL.
3.2 Different affinities of 12 MAbs demonstrated by ELISA
Purified MAbs were tested against two different antigens for their reactivity and specificity under native conditions: N-355 fragment of gp96 and full-length gp96. The results are shown in Figs.1(a) and 1(b), respectively. One particular MAb showed high affinity with N-355 fragment, even at the concentration of 0.01 μg/mL. However, of the same MAb, the reactivity to full-length gp96 was low (over 10 times lower of the OD value). Interestingly, this MAb was one of those shown negative results in Western blotting analysis. Other MAbs’ reactivities to either N-355 fragment or full-length gp96 were of the same tendency, that is, the response became weaker with lower concentration of MAbs.
3.3 Recognition of 10 out of 12 MAbs for linear epitopes determined by Western blotting
The MAbs were analyzed by Western blot for their binding specificity and reactivity with the linear epitopes of gp96 protein. Amongst all the 12 clones, 10 reacted with denatured gp96 protein, while the other 2 did not show reactivity towards the antigen (Fig.2).
3.4 Cellular gp96 detected by immunofluorescence analysis (IFA)
IFA was performed with HepG2 cells that abundantly expressed full-length gp96. As shown in Fig.3, nucleus and Rhodamine-conjugated secondary antibody were stained. Positive signals were detected around the nucleus for all the twelve MAbs. This suggested that all the MAbs can bind specifically to gp96 in its natural condition, though the binding affinities were various. Two MAbs out of the total twelve showed weaker reactivity than the others, and this in some degree was consistent with the result of flow cytometry assay (see the following section).
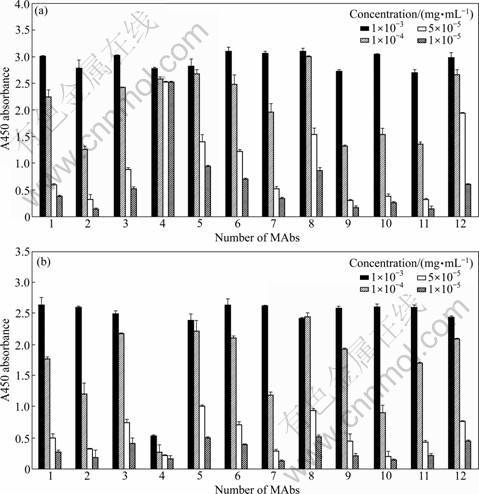
Fig.1 ELISA assay of MAbs with N-355 fragment (a) and full-length gp96 (b) respectively
![]()
Fig.2 Western blot analysis of twelve MAbs with denatured antigen

Fig.3 Detection of MAbs by immunofluorescence analysis
3.5 Human gp96 on HepG2 cells tested by flow cytometry (FCM) assay using MAbs produced
HepG2 cells were stained with these twelve different MAbs to detect whether they can bind with the natural protein. All the twelve MAbs showed binding affinity with gp96. Among all the MAbs, the percentage of fluorescent positive cells did not vary much, ranging from 96% to 99.2%, and the mean fluorescence intensity index (MFII) differed from 29.75 to 114.16 (MFII= (sample mean fluorescence intensity-control mean fluorescence intensity)/control mean fluorescence intensity) (see Table 1 as a reference). This result indicated that all the twelve MAbs can be detected to react with gp96. Figure 4 showed the representative profiles of this assay.
Table 1 Properties of twelve MAbs against full-length gp96 in ELISA and flow cytometry assay
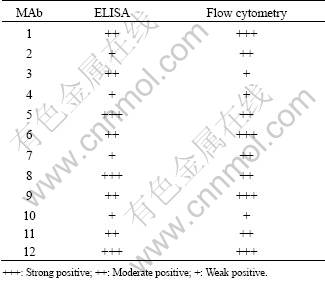

Fig.4 Flow cytometry results of HepG2 cells expressing full-length gp96
4 Discussion
In this work, we report the use of heterologously- expressed N-355 fragment of gp96 to enhance the production of monoclonal antibodies against its whole molecule after an initial immunization failure with the purified endogenous gp96, as well as the characterization of these monoclonal antibodies. Twelve monoclonal antibodies were generated and their titers, species specificities and binding affinities against swine and human gp96 were determined by ELISA, Western blot, immunofluorescence analysis and flow cytometry.
On Western blot of the denatured full-length gp96, ten out of twelve MAbs could detect the antigen protein, except for number 1 and 4 MAbs (Fig.2). However, as shown in ELISA analysis which is performed in native conditions, all of the MAbs demonstrated positive binding affinity to both full-length gp96 (extracted from liver) and N-355 fragment (expressed in E. coli) (Fig.1). These suggest that the two antibodies (number 1 and 4) may only be able to recognize non-linear epitopes, which is different from other ten MAbs.
Among these twelve antibodies, as shown in Fig.1(a), one of them (number 4) showed significantly higher affinity towards the N-355 fragment but much lower affinity towards the full-length gp96 protein. Even when it was diluted to the concentration of 1×10-5 mg/mL, the binding affinity towards the N-355 fragment did not drop much. There are two possible reasons for this. Firstly, the non-linear epitope that is recognized by number 4 MAb may be folded inside the whole gp96 molecule, but exposed to the surface of N-355 fragment. Secondly, this may be due to different expression systems of the antigens which were used to produce MAbs. As previously described, the N-355 fragment was expressed in heterologous expression system (E. coli) while full-length gp96 was either extracted from animal tissues or expressed in eukaryotic cell line (HepG2). For the full-length gp96 protein which was expressed in eukaryotic system, post-translational modifications such as glycosylation and phosphorylation can affect the surface characters of the protein. But in prokaryotic E. coli expression system, there is no post-translational modification. Different surface characters of N-355 fragment compared with full-length gp96 protein let the mouse immune system recognize it as a ‘foreign antigen’, and this explains why N-355 fragment can be used as boosts to get higher titer MAbs when full-length gp96 failed to do so. On the other hand, it will be difficult to produce any MAbs to recognize the full-length gp96 if N-355 fragment alone is used for immunization. Thus, when we first immunized the mouse with full-length gp96, and then enhanced the production of the specific monoclonal antibodies with N-355 fragment, we got some antibodies that can recognize both the full-length gp96 protein and N-355 fragment. This has some implications for the production of MAbs in mice with sequence-conserved proteins between mice and other species.
Applications of immunofluorescence and FCM analyses in HepG2 cell line further characterized the features of MAbs, and they suggested that all the MAbs could bind to natural gp96 with different affinities (Fig.3 and Fig.4). For instance, number 4 monoclonal antibody showed the smallest fluorescence intensity in IFA analysis and lowest binding affinity in FCM assay, while number 12 monoclonal antibody showed strong reactivity in both of the analyses.
The twelve monoclonal antibodies we produced can be applied in functional and structural studies in the future (Available upon asking, gaof@im.ac.cn). For instance, in further functional studies, these monoclonal antibodies can be used as blockade antibodies to inhibit the functions of gp96. Also, they can be used as assistant molecules to facilitate co-crystallization with native gp96 protein to investigate its whole structure.
Our method has provided a new solution of producing monoclonal antibodies against some sequence-conserved proteins between mice and other mammalians with low immunogenicity. It results in getting a batch of MAbs at the same time, targeting at linear or conformational epitopes. It also solves the shortages of other approaches to some degree, for example, priming and boosting with synthetic small poly-peptides might increase the cost of antibody production; using recombinant protein as one and only immunogen may lead to the defect that generated antibodies will not be able to recognize eukaryotic antigen. Compared with them, our method not only reduces the cost and workload, as well as simplifies the procedure, but also has the potential to provide a wider range of choices of antibodies that can bind to cellular antigen in its natural condition. All of the above characteristics make our strategy a practical and suitable option for mass production of antibodies against low-immunological and highly-conserved antigens.
Acknowledgement
We thank the staff in the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences for their technical support in producing monoclonal antibodies. GFG is a leading principal investigator of the Innovative Research Group of the National Natural Science Foundation of China (NSFC, Grant No. 81021003).
References
[1] SHIU R P, POUYSSEGUR J, PASTAN I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts [J]. Proc Natl Acad Sci USA, 1977, 74: 3840-3844.
[2] GUPTA R S. Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species [J]. Mol Biol Evol, 1995, 12: 1063-1073.
[3] CHAVANY C, MIMNAUGH E, MILLER P, BITTON R, NGUYEN P, TREPEL J, WHITESELL L, SCHNUR R, MOYER J, NECKERS L. p185erbB2 binds to GRP94 in vivo. Dissociation of the p185erbB2/GRP94 heterocomplex by benzoquinone ansamycins precedes depletion of p185erbB2 [J]. J Biol Chem, 1996, 271: 4974-4977.
[4] FERREIRA L R, NORRIS K, SMITH T, HEBERT C, SAUK J J. Hsp47 and other ER-resident molecular chaperones form heterocomplexes with each other and with collagen type IV chains [J]. Connect Tissue Res, 1996, 33: 265-273.
[5] MELNICK J, AVIEL S, ARGON Y. The endoplasmic reticulum stress protein GRP94, in addition to BiP, associates with unassembled immunoglobulin chains [J]. J Biol Chem, 1992, 267: 21303-21306.
[6] MELNICK J, DUL J L, ARGON Y. Sequential interaction of the chaperones BiP and GRP94 with immunoglobulin chains in the endoplasmic reticulum [J]. Nature, 1994, 370: 373-375.
[7] SCHAIFF W T, HRUSKA K A, MCCOURT D W Jr, GREEN M, SCHWARTZ B D. HLA-DR associates with specific stress proteins and is retained in the endoplasmic reticulum in invariant chain negative cells [J]. J Exp Med, 1992, 176: 657-666.
[8] WASSENBERG J J, REED R C, NICCHITTA C V. Ligand interactions in the adenosine nucleotide-binding domain of the Hsp90 chaperone, GRP94: II. Ligand-mediated activation of GRP94 molecular chaperone and peptide binding activity [J]. J Biol Chem, 2000, 275: 22806-22814.
[9] LEE A S. Mammalian stress response: Induction of the glucose- regulated protein family [J]. Curr Opin Cell Biol, 1992, 4: 267-273.
[10] SRIVASTAVA P K, UDONO H, BLACHERE N E, LI Z. Heat shock proteins transfer peptides during antigen processing and CTL priming [J]. Immunogenetics, 1994, 39: 93-98.
[11] STRBO N, PODACK E R. Secreted heat shock protein gp96-Ig: An innovative vaccine approach [J]. Am J Reprod Immunol, 2008, 59: 407-416.
[12] CSERMELY P, SCHNAIDER T, SOTI C, PROHASZKA Z, NARDAI G. The 90-kDa molecular chaperone family: Structure, function, and clinical applications. A comprehensive review [J]. Pharmacol Ther, 1998, 79: 129-168.
[13] ARNOLD D, FAATH S, RAMMENSEE H, SCHILD H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96 [J]. J Exp Med, 1995, 182: 885-889.
[14] SRIVASTAVA P K, UDONO H. Heat shock protein-peptide complexes in cancer immunotherapy [J]. Curr Opin Immunol, 1994, 6: 728-732.
[15] SCHILD H, RAMMENSEE H G. gp96—The immune system’s Swiss army knife [J]. Nat Immunol, 2000, 1: 100-101.
[16] SINGH-JASUJA H, HILF N, SCHERER H U, ARNOLD-SCHILD D, RAMMENSEE H G, TOES R E, SCHILD H. The heat shock protein gp96: A receptor-targeted cross-priming carrier and activator of dendritic cells [J]. Cell Stress Chaperones, 2000, 5: 462-470.
[17] MENG S D, GAO T, GAO G F, TIEN P. HBV-specific peptide associated with heat-shock protein gp96 [J]. Lancet, 2001, 357: 528-529.
[18] MENG S D, SONG J, RAO Z, TIEN P, GAO G F. Three-step purification of gp96 from human liver tumor tissues suitable for isolation of gp96-bound peptides [J]. J Immunol Methods, 2002, 264: 29-35.
[19] SRIVASTAVA P K, DELEO A B, OLD L J. Tumor rejection antigens of chemically induced sarcomas of inbred mice [J]. Proc Natl Acad Sci USA, 1986, 83: 3407-3411.
[20] LI H, ZHOU M, HAN J, ZHU X, DONG T, GAO G F, TIEN P. Generation of murine CTL by a hepatitis B virus-specific peptide and evaluation of the adjuvant effect of heat shock protein glycoprotein 96 and its terminal fragments [J]. J Immunol, 2005, 174: 195-204.
[21] WARGER T, HILF N, RECHTSTEINER G, HASELMAYER P, CARRICK D M, JONULEIT H, VON LANDENBERG P, RAMMENSEE H G, NICCHITTA C V, RADSAK M P, SCHILD H. Interaction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responses [J]. J Bio Chem, 2006, 281: 22545-22553.
[22] LIU S, TOBIAS R, MCCLURE S, STYBA G, SHI Q, JACKOWSKI G. Removal of endotoxin from recombinant protein preparations [J]. Clin Biochem, 1997, 30: 455-463.
[23] FERRARINI M, HELTAI S, ZOCCHI M R, RUGARLI C. Unusual expression and localization of heat-shock proteins in human tumor cells [J]. Int J Cancer, 1992, 51: 613-619.
[24] ZHU X D, LI C L, LANG Z W, GAO G F, TIEN P. Significant correlation between expression level of HSP gp96 and progression of hepatitis B virus induced diseases [J]. World J Gastroenterol, 2004, 10: 1141-1145.
(Edited by YANG Bing)
Foundation item: Project(31030030) supported by the National Natural Science Foundation of China
Received date: 2011-05-06; Accepted date: 2011-09-22
Corresponding author: GAO George Fu, Professor; Tel: +86-10-64807688; Fax: +86-10-64807882; E-mail: gaof@im.ac.cn
Abstract: Gp96, a member of HSP90 family, is a versatile molecular chaperone with various newly-discovered functions, for example to serve as a low affinity, high capacity calcium binding protein, a natural adjuvant for therapeutic cancer vaccines, a tumor rejection antigen, an immune regulator to pathological cell death. Its multi-functional and structural characteristics make it also an interesting target to develop antibody-based therapeutics. However, its low immunogenicity to mice, because of its high-sequence similarity among different species, is an obstacle to obtain valuable monoclonal antibodies (MAbs). This is a common problem for any low immunogenic proteins, whose sequences share close identity between mice and other species. Here, a new strategy of priming was employed by swine endogenous full-length gp96 and then boosting by E. coli-system heterologously expressed gp96 N-terminal fragment (N-355) to generate MAbs. Twelve different highly-specific MAbs against swine/human endogenous gp96 were successfully obtained. The binding activities of these MAbs were confirmed by enzyme-linked immunosorbent assay (ELISA), Western blot (WB), immunofluorescence and flow cytometry analysis. This provides some important reagents for further research and potential therapeutics. The methods employed can be used for MAb production of any sequence-highly-conserved proteins between mice and swine/human (or any other species).


