
Effects of heat treatment on characteristics of porous Ni-rich NiTi SMA prepared by SHS technique
CHU Cheng-lin(储成林)1, CHUNG Jonathan-CY(钟志源)2, CHU Paul-K(朱剑豪)2
1. Department of Materials Science and Engineering, Southeast University, Nanjing 210096, China;
2. Department of Physics and Materials Science, City University of Hong Kong, Hong Kong, China
Received 8 March 2005; accepted 4 July 2005
Abstract:
The effects of heat treatment on the microstructure and compressive properties of porous Ni-rich NiTi shape memory alloy (SMA) fabricated by self-propagating high-temperature synthesis (SHS) were investigated. The solution treatment at 1050℃ has little effects on stable Ti2Ni second phase, however, it decreases the amount of Ni4Ti3 phase derived from the SHS process and results in the improvement of the ductility of porous NiTi SMA. The subsequent aging treatment after solution treatment could lead to the precipitation of the discrete Ni4Ti3 phase in NiTi matrix grains, which increases the brittleness of porous NiTi SMA. Porous NiTi SMA presents a composite fracture behavior consisting of a ductile fracture of NiTi matrix and a cleavage fracture of second phase particles. Many cracks existing on the interfaces indicate that the bonding of the matrix with second phase particles is weak.
Key words:
NiTi shape memory alloy; porous material; self-propagating high-temperature synthesis; microstructure; compressive properties;
1 Introduction
Porous NiTi SMA whose mechanical properties can be easily adjusted to match those of replaced hard tissues by obtaining different porosity and pore sizes through controlling the synthesis conditions has been acknowledged as a promising biomaterial for use as artificial bones or teeth roots[1-4]. Moreover, its porous structure could allow body tissues to grow inside and body fluids to be transported through the interconnected pores. Self-propagating high-temperature synthesis(SHS) technique has been proven to be an ideal alternative to produce porous NiTi SMAs by igniting a compressed powder mixture of Ti+Ni in an inert atmosphere and producing a self-sustaining chemical reaction with sufficient heat released. It has the advantages of saving time and energy over powder metallurgy[5-12]. Up to now, many porous NiTi SMAs with different pore structures were developed by SHS method. CHU et al[5] fabricated porous NiTi SMA with homogeneously distributing spherical pores and porosity of 70% by SHS. LI et al[6-8] prepared porous NiTi SMA with the anisotropic pore structure successfully by SHS. The pores in the porous NiTi SMA with porosity of about 57%-68% are three- dimensionally interconnected. CHU and CHUNG[9-12] recently developed porous NiTi SMA with the three-dimensionally interconnected isotropic pore structure by an improved SHS method.
Porous NiTi SMAs fabricated by SHS have the common feature of composition inhomogeneity because the raw powders are mixed insufficiently and the reactant particle is not small enough[6-13]. As a result, porous NiTi SMAs usually have the complicated microstructure, which has important effects on their properties. The microstructure and properties of porous NiTi SMAs can be modified by heat treatment. However, few systematic literatures have been reported to date to study the effects of heat treatment on the microstructure and properties of porous NiTi SMAs, especially porous Ni-rich NiTi SMAs prepared by SHS.
In this paper, porous Ni-rich NiTi SMA was fabricated by SHS. Then the effects of heat treatment on the microstructure and compressive properties of porous Ni-rich NiTi SMA were investigated using optical microscope, scanning electron microscope(SEM), energy- dispersive analysis by X-rays facility(EDAX), X-ray diffraction(XRD) and Instron testing machine.
2 Experimental
Porous Ni-rich NiTi SMA was produced by the SHS technique as described in Ref.[9]. The mixed powders of Ni(99.5%, mass fraction, the same below if not mentioned) and Ti (99.3%) with 51% Ni(mole fraction) were blended by ball milling for 12 h, and then pressed into cylindrical compacts of 50 mm in diameter and 25 mm in height. The pressure used was sufficient to give a green density of (45±2)% theoretical value. A small hole was drilled in the side of the compact to accommodate the W-5%Re/W-26%Re thermocouple. The temperature—time profile was recorded during the furnace heating of the sample using a strip chart recorder. The preheating temperature was 350 ℃. The preheated pellet was ignited at one end by an ignition reagent (4 g in mass) composed of Ti and C powders with 50% Ti(mole fraction) under an atmosphere pressure (about 0.1 MPa) of 99.98% pure argon flowing. Once ignited, combustion wave could self-propagate along the axis to the other end of the compact, and then porous NiTi SMA was synthesized.
Pore characteristics of porous NiTi SMA were analyzed with optical microscope. The general porosity was determined by mass and dimensional measurements. Porous NiTi SMA was treated by solution treatment at 1050℃ for different time and subsequent aging treatment at different temperatures for 1 h. A flowing argon environment was used in the furnace to protect the samples from oxidizing. The samples were etched for 10 s in a mixture of 5% HNO3, 10% HF in water. Then the microstructure was examined using optical microscope and scanning electron microscope (SEM). The phase constituent and chemical compositions of the specimens were determined by X-ray diffraction (XRD) analysis and energy-dispersive analysis by X-ray facility (EDAX).
The compressive samples were cut in a size of about 6mm in width, 5 mm in thickness and 15mm in highness. Uniaxial compression test was carried out at a constant rate of 0.1 mm/min on Instron testing machine (4400 type) to investigate the compressive properties of porous NiTi SMA at room temperature. The compressive strength was defined as the maximum value in the stress—strain curve, and the compressive strain was the value corresponding to the compressive strength in the stress-strain curve. The average compressive properties are obtained by repeating the compressive experiments with at least five different samples. The fracture surfaces of the samples were observed by SEM in order to determine their fracture behaviors.
3 Results and discussion
Porous Ni-rich NiTi SMA fabricated in this work has an average porosity of 57.3%(volume fraction) with a deviation of ±1%. Optical micrograph (Fig.1) shows that the size of the pores in porous NiTi SMA is about 200—500 mm and most pores are three-dimensionally interconnected. Porous NiTi SMA has the pore structure with an isotropic feature in the morphology and distribution of the pores. Fig.2(a) shows the XRD pattern of porous NiTi SMA without any heat treatment. Besides B2(NiTi) parent phase and B19’(NiTi) martensitic phase, two second phases including Ni-rich Ni4Ti3 and Ti-rich Ti2Ni exist in the sample. No Ni-rich Ni3Ti phase could be observed. Fig.2(b) and c show XRD patterns of porous NiTi SMAs after solution treated at 1 050 ℃ for 2 and 4 h, respectively. It is obvious that Ti2Ni phase could not be removed by solid-state diffusion under the given conditions.
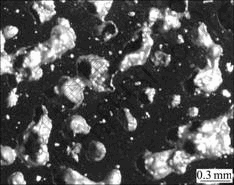
Fig.1 Optical micrograph of SHS-synthesized porous Ni-rich NiTi SMA
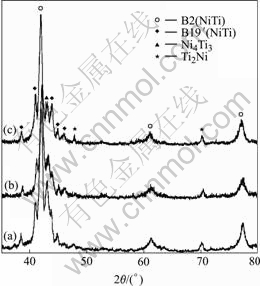
Fig.2 XRD patterns of porous Ni-rich NiTi SMAs after solution treated at 1 050 ℃ for different time: (a) Non-treated; (b) 2 h; (c) 4 h
According to an equilibrium diagram of Ti-Ni system[14], Ti2Ni is thermodynamically stable above 1 050 ℃, while Ni4Ti3 is metastable under the same temperature. The combustion temperature of Ni+Ti system recorded by the thermocouple in this work is up to 1 310 ℃, thus it is possible that the formation of Ti2Ni is prior to that of Ni4Ti3 during the fabricating process of SHS. As a result, the presence of Ti-rich Ti2Ni phase in Ni-rich NiTi SMA(51% Ni, mole fraction) could make NiTi parent phase more Ni-rich. Thus the extra nickel then forms Ni-rich Ni4Ti3 phase. This may also be the reason why the solution treatment at 1 050 ℃ for a short time (less than 4 h) could not remove metastable Ni4Ti3 phase formed during the SHS process completely. However, the amount of Ni4Ti3 phase in porous NiTi SMA decreases slightly after solution treatment, which can improve the ductility of porous NiTi SMA. As indicated by Table 1, the compressive strain and compressive strength of the sample non-treated are about 4.8% and 208 MPa, respectively. After porous NiTi SMA is treated at 1 050 ℃ for 4 h, the compressive strain increases to 6.2%, while the compressive strength decreases to 173 MPa.
Table 1 Average compressive properties of porous Ni-rich NiTi SMAs with porosity of 57.3% after different heat treatments
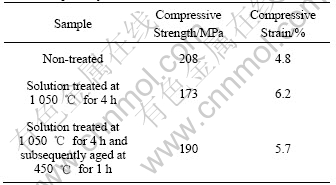
Fig.3 shows XRD patterns of porous Ni-rich NiTi SMAs after solution treatment at 1 050 ℃ for 4 h and subsequent aging treatment at different temperatures for 1 h. It could be found that the subsequent aging treatment can increase the amount of Ni4Ti3 phase in porous NiTi SMA. Fig.4(a) shows the optical micrograph of the microstructure of porous Ni-rich NiTi SMA after solution treated at 1 050 ℃ for 4 h. The dark discrete phase is Ti2Ni, confirmed as Li2Ni using an energy-dispersive analysis by X-ray (EDAX) facility, which often occurs near grain boundaries. The white network-like phase in the gray NiTi matrix is Ni4Ti3, which is derived from the fabricating process of SHS. Fig.4(b) and Fig.5 show the optical and SEM micrographs of porous Ni-rich NiTi SMA after solution treatment at 1 050 ℃ for 4 h and subsequent aging treatment at 450 ℃ for 1 h, respectively. It can be found that besides the network-like Ni4Ti3 phase deriving from the fabricating process of SHS, many white Ni4Ti3 particles were discretely precipitated in NiTi matrix grains during the aging process (Figs.4(b) and 5(a)). In addition, the needle-like B19’(NiTi) martensitic phase in porous NiTi SMA is also found by SEM observation with a high magnification (Fig.5(b)).
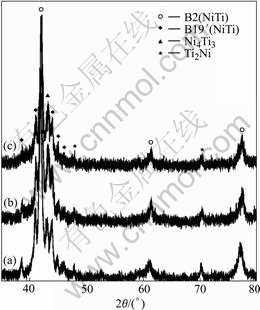
Fig.3 XRD patterns of porous Ni-rich NiTi SMAs after solution treated at 1 050 ℃ for 4 h and subsequent aging treated at different temperatures for 1 h: (a) Non-aging; (b) Aging at 400 ℃; (c) Aging at 450 ℃
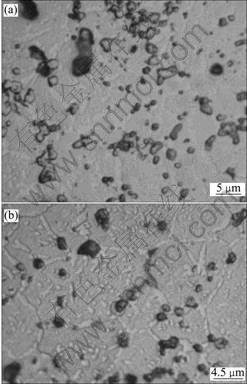
Fig.4 Optical micrographs of porous Ni-rich NiTi SMAs: (a) After solution treated at 1 050 ℃ for 4 h; (b) After solution treated at 1 050 ℃ for 4 h and subsequent aged at 4 50 ℃ for 1 h
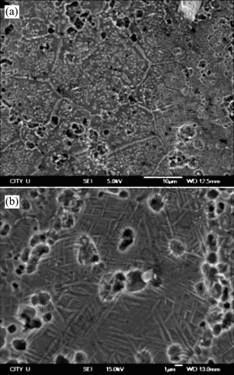
Fig.5 SEM micrographs of porous Ni-rich NiTi SMA after solution treatment at 1 050 ℃ for 4 h and subsequent aging treatment at 450 ℃ for 1 h: (a) Typical morphology; (b) Marten- sitic morphology
It is well known that the precipitation of Ni4Ti3 phase in NiTi matrix during the aging process can affect the features of martensitic transformation in Ni-rich NiTi SMA by promoting the formation of R-phase prior to the formation of B19’ from B2 matrix on cooling[14, 15]. The coherency stress fields develop due to the different lattice parameter and crystal structure of Ni4Ti3 (rhombohedral) precipitate with the matrix (B2), which can act as the strong resistance to large lattice variant deformations (on the order of 10%) associated with the formation of B19’. The initial transformation from B2 to R-phase with a very small transformation strain about 1/10 of that in B2→B19’ transformation can reduce the overall energy of the system[15]. Further cooling then transforms the resulting R-phase to B19’ because B19’ is more thermodynamically stable than R-phase. It should be noted that this precipitation of Ni4Ti3 phase in NiTi matrix during the aging process could increase the brittleness of porous NiTi SMAs. As shown by Table 1, the compressive strain decreases slightly from 6.2% of the sample after solution treatment at 1 050 ℃ for 4 h to 5.7% of the sample after solution treated at 1 050 ℃ for 4 h and subsequently aged at 450 ℃ for 1 h.
The fracture surface characteristics of porous Ni-rich NiTi SMA after solution treatment at 1 050 ℃ for 4 h and aging treatment at 450 ℃ for 1 h is shown in Fig.6. It can be found that the porous NiTi SMA has a composite fracture characteristics composed of ductile fracture with some ductile dimples and brittle cleavage fracture, namely NiTi matrix shows a ductile fracture behavior, while the second phase particles in the matrix present a cleavage fracture. There are many cracks existing on the interfaces between the matrix and the second phase particles, which indicate clearly that the bonding of the matrix with the second phase particles is poor.
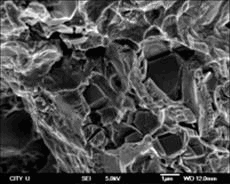
Fig.6 SEM fractograph of porous Ni-rich NiTi SMA after solution treatment at 1 050 ℃ for 4 h and aging treatment at 450 ℃ for 1 h
4 Conclusions
Besides NiTi matrix phase (B2 and B19’), two second phases including Ni4Ti3 and Ti2Ni are present in porous Ni-rich NiTi SMA prepared by SHS method. The solution treatment at 1 050 ℃ has little effect on stable Ti2Ni phase, however, it decreases the amount of Ni4Ti3 phase and results in the improvement of the ductility of porous NiTi SMA. The subsequent aging treatment after solution treatment can lead to the precipitation of the discrete Ni4Ti3 phase in NiTi matrix grains, which increases the brittleness of porous NiTi SMA. NiTi matrix shows a ductile fracture behavior, while second phase particles present a cleavage fracture. Many cracks existing on the interfaces indicate that the bonding of the matrix with second phase particles is weak.
References
[1] Itin V I, Gjunter V E, Shabalovskaya S A, Sachdeva R L C. Mechanical properties and shape memory of porous Nitinol[J]. Materials Characterization, 1994, 32(3): 179-187.
[2] Lipscomb I P, Nokes L D M. The Application of Shape Memory Alloys in Medicine[M]. Suffold: Mechanical Engineering Publications Limited, 1996.
[3] Kujalaa S, Ryhanena J, Danilovb A, Tuukkanen J. Effect of porosity on the osteointegration and bone ingrowth of a weight-bearing nickel–titanium bone graft substitute[J]. Biomaterials, 2003, 24(25): 4691–4697.
[4] Gjunter V E. Superelastic Shape Memory Implants in Maxillofacial Surgery, Traumatology, Orthopaedics and Neurosurgery[M]. Tomsk: Tomsk University Publishing House(TUP), 1995.
[5] Chu C L, Li B, Wang S D, Zhang S G, Yin Z D. Preparation of TiNi shape memory alloy porosint by SHS[J]. Trans Nonferrous Met Soc China, 1997, 7(4): 84-87.
[6] Li B Y, Rong L J, Li Y Y, Gjunter V E. Synthesis of porous Ni–Ti shape memory alloys by self-propagating high-temperature synthesis: reaction mechanism and anisotropy in pore structure[J]. Acta Mater, 2000, 48(15): 3895–3904.
[7] Li B Y, Rong L J, Li Y Y, Gjunter V E. An investigation of the synthesis of Ti-50at%Ni alloys through combustion synthesis and conventional powder sintering[J]. Metall Trans A, 2000, 31(7): 1867-1871.
[8] Li B Y, Rong L J, Li Y Y, Gjunter V E. Novel electric resistance phenomena in porous Ni-Ti shape memory alloys produced by SHS[J]. Scripta Materialia, 2001, 44(5): 823-827.
[9] Chu C L, Chung C Y, Lin P H, Wang S D. Fabrication of porous NiTi shape memory alloy for hard tissue implants by combustion synthesis[J]. Mater Sci Eng A, 2004, 366(1): 114-119.
[10] Chung C Y, Chu C L, Wang S D. Porous NiTi shape memory alloy with high strength fabricated by self-propagating high- temperature synthesis[J]. Materials Letters, 2004, 58(11): 1683-1686.
[11] Chu C L, Chung C Y, Lin P H. Characterization of transformation behavior in porous Ni-rich NiTi shape memory alloy fabricated by combustion synthesis[J]. J Mater Sci, 2005, 40(3): 773-776.
[12] Chu C L, Chung C Y, Lin P H. Phase transformation behaviors in porous Ni-rich NiTi shape memory alloy fabricated by combustion synthesis[J]. Mater Sci Eng A, 2005, 392(1-2): 106-111.
[13] Yi H C, Moore J J. Combustion synthesis of TiNi intermetallic compounds(Part III): Microstructural characterization[J]. J Mater Sci, 1992, 27(18): 5067-5072.
[14] Otsuka K, Wayman C M. Shape Memory Materials[M]. Cambridge: Cambridge University Press, 1998.
[15] Ren X, Miura N, Zhang J, Otsuka K, Tanaka K, Koiwa M, Suzuki T, Chumlyakov Y I, Asai M. A comparative study of elastic constants of Ti–Ni-based alloys prior to martensitic transformation[J]. Mater Sci Eng A, 2001, A312(1-2): 196-206.
Foundation item: Project(CityU 1/04C) supported by Hong Kong Grants Council Central Allocation Group Research Project; Project(50501007) supported by the National Natural Science Foundation of China; Project(BK2003062) supported by the Natural Science Foundation of Jiangsu Province, China; Project(4012001007) supported by the Teaching and Research Award Program for Outstanding Young Teachers of Southeast University; Project(9212001352) the Pre-research Project for National Natural Science Foundation in Southeast University
Corresponding author: CHU Cheng-lin; Tel: +86-25-83996387; E-mail: clchu@seu.edu.cn
(Edited by LONG Huai-zhong)


