采用非均相沉淀-热还原法制备铁包覆氮化硅复合粉末
银锐明1, 2, 范景莲1,刘 勋1,黄伯云1
(1. 中南大学 粉末冶金国家重点实验室,湖南 长沙,410083;
2. 湖南工业大学 焊接研究所,湖南 株洲,412008)
摘 要:
摘 要:以FeSO4?7H2O,Na2CO3和Si3N4为原料,聚乙二醇400为分散剂,采用非均相沉淀-热还原法制备Fe/Si3N4金属陶瓷复合粉末,将未经表面导电处理的Fe/Si3N4金属陶瓷复合粉末与Si3N4粉末进行SEM扫描比较分析及EDS,XRD和Zeta电位测量等方法表征并对微观结构进行讨论。研究结果表明,Si3N4被连续、致密、均匀的SiOxNy,SiO2和Fe三层壳体层层包覆,壳与壳之间化学键结合紧密。
关键词:
中图分类号:TB 333 文献标识码:A 文章编号:1672-7207(2008)01-0007-05
Preparation of Fe-coated Si3N4 composite powder by heterogeneous precipitation-thermal reduction process
YIN Rui-ming1, 2, FAN Jing-lian1, LIU Xun1, HUANG Bai-yun1
(1. State Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, China;
2. Institute of Welding Research, Hunan University of Technology, Zhuzhou 412008, China)
Abstract: Using FeSO4.7H2O, NaCO3 and Si3N4 as raw materials and poly(ethylene glycol)-400 as dispersant, Fe-coated Si3N4 composite powder was synthesized by the heterogeneous precipitation-thermal reduction process. SEM of Fe/Si3N4 composite powder and Si3N4 without conductive coating were compared, and the structures of Fe/Si3N4 composite powder were characterized by EDS, XRD and zeta potential analysis, and the microstructure of Fe/Si3N4 composite powder was discussed. The results show that the surface of Si3N4 is consistently, compactly and uniformly coated by layers of SiOxNy, SiO2 and Fe, and the bonding between layers and extensive strong chemical bonds is very firm.
Key words: coating; Fe; Si3N4; heterogeneous precipitation
金属陶瓷作为一种金属/陶瓷复合材料既具有陶瓷材料的高强度、高硬度等优点,又具有金属材料的韧性和可加工性,逐渐成为材料改性的主要材料[1-2]。Si3N4高温陶瓷在高温领域比较其他高温陶瓷具有明显优势。如与Si3N4高温陶瓷相比,氧化铝陶瓷耐热冲击差,强度低;氧化锆陶瓷高温强度低,硬度低;碳化硅陶瓷的抗弯强度只达到氮化硅陶瓷的一半。金属Fe与其他金属相比具有中等熔点与很强的强度和韧性,与陶瓷复合能有效提高陶瓷的脆性,并能承受一定的高温。人们将金属Fe与 Si3N4复合制备金属陶瓷进行了大量研究[3-11],但制备方法均采用的是传统的粉末冶金法、原位合成法和熔融浸渍法,虽工艺简单,但不能保证Fe均匀分散于Si3N4中,而强韧化金属陶瓷的关键是金属相均匀分散在陶瓷相中。研究结果表明[12],金属包裹法能将超细或纳米金属颗粒沉积在陶瓷颗粒表面,使金属均匀分散在陶瓷之中,烧结过程中各向界面反应特性相同,晶粒生长一致,大大提高了显微结构的均匀性,使陶瓷粉体能在最大程度上均匀分布在金属三维结构中[13]。在此,本文作者采用非均相成核-热还原法,制备Fe均匀包覆Si3N4颗粒的复合粉末。
1 实 验
以工业生产的氮化硅以及分析纯FeSO4?7H2O和Na2CO3为原料,纯聚乙二醇400为分散剂。配制FeSO4水溶液浓度为0.15 mol/L,配制Na2CO3水溶液浓度为0.3 mol/L,Si3N4悬浮液质量浓度为15 g/L。超声波震荡及搅拌器搅拌,加入适量聚乙二醇400的Si3N4悬浮液10 min后,将配制好的FeSO4与Na2CO3水溶液同时缓缓加入,再经超声波震荡及搅拌器搅拌10 min,静置过滤,在80 ℃的温度下烘干,于室温下手工研磨,得前驱体粉末。
将前驱体粉末用去离子水反复清洗几次,烘干置入铁容器中,推入氢气还原炉中还原,升温速度为5 ℃/min,在800 ℃保温1 h,冷却室温后经手工研磨,得Fe包覆Si3N4的复合粉末。
表征检测设备有:日本理学电机株式会社的D/max 2550型XRD衍射仪,Cu Kα辐射,连续扫描方式采样,扫描速度为4(?)/min;二次电子成像模式;JEOL公司的JSM-6360LV型电子扫描显微镜和LEDAX公司的EDX-GENESIS 60S型能谱仪。
2 结果与讨论
2.1 制备过程分析
FeSO4溶液与Na2CO3溶液混合反应生成黄绿色FeCO3沉淀[14-15]。而非均相成核原理是异质相加入时,在一定程度上降低成核的能量势垒,诱导晶核的生成,在适宜条件下,溶液反应生成的沉淀物以异质相为核心长大,最终获得反应生成物均匀包覆异质相表面,形成核壳结构的复合颗粒。因此,将FeSO4溶液与Na2CO3溶液同时缓缓倒入Si3N4悬浮液时,FeSO4与Na2CO3反应生成的FeCO3被吸附在Si3N4上并长大,形成FeCO3包覆Si3N4的复合颗粒。
在温度为80 ℃的烘干过程中,Fe/Si3O4复合粉末颜色由黄绿色转变为朱红色,对其进行X射线衍射,结果如图1所示。图中显示只有Fe2O3,Si3N4和Na2SO4的衍射峰,由此可认为在烘干过程中包覆Si3N4的FeCO3与空气中O2发生反应,生成了朱红色Fe2O3,获得Fe/Si3N4复合粉末的前驱体。上述实验过程的基本反应方程式如下:
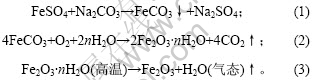
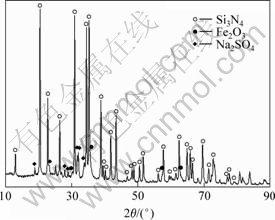
图1 Fe/Si3N4复合粉末前驱体XRD图谱
Fig.1 XRD pattern of Fe/Si3N4 composite powder precursor
在800 ℃时,氢气还原前驱体粉末,对Fe/Si3O4复合粉末进行X射线衍射分析,结果如图2所示。从图2可见,只有Fe与Si3N4的衍射峰,没有铁的氧化物的衍射峰,由此表明,在800 ℃和氢气气氛下,Fe氧化物被还原,还原产物 Fe与Si3N4没有或仅发生了极弱小的界面反应,由此判断所得粉末为Fe/Si3N4复合粉末。
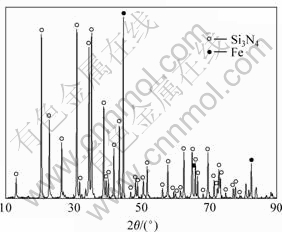
图2 Fe/Si3N4复合粉末的XRD图谱
Fig.2 XRD pattern of Fe/Si3N4 composite powder
2.2 表征分析
对没有进行表面导电处理的Si3N4粉末与Fe/Si3N4复合粉末进行SEM扫描,结果如图3和图4所示。图3所示图像模糊不清,图4所示图像清晰,其原因是:Si3N4是绝缘体,在进行SEM扫描时,电子束扫射在绝缘体或导电性能差的样品上,形成电子堆积,阻挡入射电子束进入和样品内激发的二次电子逸出样品表面,造成成像不清晰[16]。而制备Fe/Si3N4复合粉末时导电的铁金属连续、致密地包覆在Si3N4表面,从而相当于在Si3N4表面镀上一层导电层,故SEM扫描成像清晰。

图3 未喷金的Si3N4的SEM照片
Fig.3 SEM photograph of Si3N4 powder without coating gold
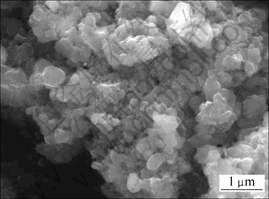
图4 未喷金的Fe/Si3N4复合粉末SEM照片
Fig.4 SEM photograph of Fe/Si3N4 composite powder without coating gold
对进行了表面导电处理即喷金的Si3N4粉末与Fe/Si3N4复合粉末进行SEM扫描,结果如图5和图6所示。可以看出,Si3N4颗粒与Fe/Si3N4复合粉末颗粒粒径相差不大,但Fe/Si3N4复合粉末颗粒表面相对不够平滑,棱角不分明,形状略接近球形,经分析认为是薄层Fe包覆所致。对图4中某一颗粒进行EDS线扫描分析,结果见图7。从图7可见,Fe,Si和N元素在扫描线上均有分布。对其他颗粒进行EDS线分析,结果与之类似。Fe元素在扫描线上分布表明铁金属不是以单独的颗粒分散分布,而是连续地沉积包覆在Si3N4表面。
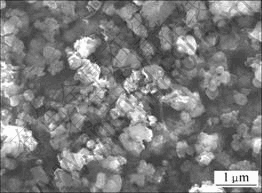
图5 喷金的Si3N4粉末SEM照片
Fig.5 SEM photograph of Si3N4 powder with coating gold

图6 喷金的Fe/Si3N4复合粉末SEM照片
Fig.6 SEM photograph of Fe/Si3N4 composite powder with coating gold
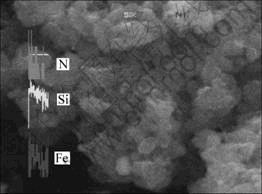
图7 Fe/Si3N4复合粉末的EDS线扫描分析结果
Fig.7 EDS line scan results of Fe/Si3N4 composite powder
将Si3N4粉末与Fe/Si3N4复合粉末悬浮液分别进行Zeta电位测试。结果见图8和图9。可见,Si3N4表面呈电负性,其Zeta电位平均值为-15.81 mV;Fe/Si3N4复合粉末表面呈电负性,其Zeta电位平均值为-1.967 mV。可见,Si3N4被铁包覆前后Zeta电位发生了变化,Zeta电位实际表征的是颗粒表面带电荷的情况,Fe的包覆使其颗粒表面的电荷发生了改变,这表明Fe包覆了Si3N4。
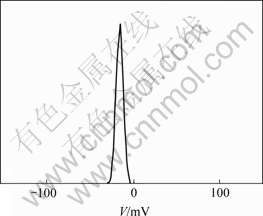
图8 Si3N4粉末的Zeta电位
Fig.8 Zeta potential of Si3N4 powder
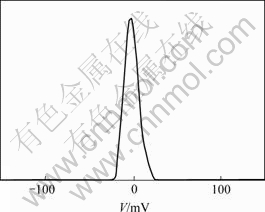
图9 Fe/Si3N4复合粉末的Zeta电位
Fig.9 Zeta potential of Fe/Si3N4 composite powder
2.3 微结构讨论
Si3N4在氧化过程中,表面形成SiO2层。目前,人们普遍认为,在氧化的初始阶段和较低的温度下,氧化层处于非晶态。徐常明等[17]发现Si3N4对SiO2析晶有明显的抑制作用;QI等[18]发现在约800 ℃的还原气氛下以CVI方法制备的3D-SiO2f/Si3N4复合材料,分别在1 400 ℃和1 600 ℃处理后无析晶现象发生。本文作者所制备的Fe/Si3N4复合粉末在氢气气氛中于800 ℃烧结1 h后Si3N4表面被非晶态Si氧化物层包覆。在图4基础上对Fe/Si3N4进行面扫描,结果见图10。从图10可见,Fe含量与加入的理论计算值相差不大。而Si和N元素含量比远大于Si3N4的原子配比3?4,这表明Si3N4表面存在非晶态Si氧化物,其使Si3N4表面至有限深度内的Si的原子分数偏大,N原子分数偏少。
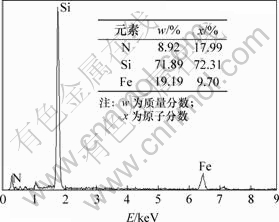
图10 Fe/Si3N4复合粉末EDS面扫描分析结果
Fig.10 EDS planescan results of Fe/Si3N4 composite powder
Ogbuji等[19-20]采用AES和RBS等方法进行分析,发现SiO2与Si3N4之间存在一种无定形的固溶连续SiNxOy化合物,其成分由SiO2连续变化到Si3N4。可见,Si3N4-SiNxOy-SiO2层之间没有十分明显的界面,通过化学键的方式紧密地结合在一起。姚红英等[21]的计算结果表明,Fe原子与SiO2结合能达到了3.34 eV,其原因是Fe与SiO2表面的Si和O形成了5个化学键。图11所示为Fe原子在SiO2表面的局域结构。可见,所制备的Fe/Si3N4复合颗粒结构为Si3N4颗粒,其表面分别被很薄的SiOxNy,SiO2和Fe壳体连续、致密地层层包裹,形成层与层之间化学键结合紧密的“芯-壳”微结构,如图12所示。
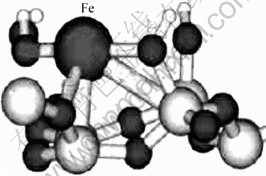
图11 Fe原子在SiO2表面的局域结构
Fig.11 Microstructure of Fe atoms adsorbed on SiO2
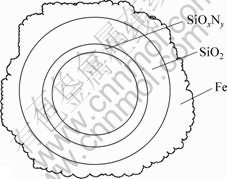
图12 Fe/Si3N4复合颗粒微结构
Fig.12 Microstructure of Fe/Si3N4 composite particle surface
3 结 论
a. 采用非均相成核法,即异质相Si3N4形核, FeSO4?7H2O与Na2CO3反应生成物FeCO3包覆长大,空气中高温氧化获得Fe包覆Si3N4颗粒的前驱体。
b. 前驱体在800 ℃和氢气气氛下还原1 h,Fe全部被还原,Fe与Si3N4没有发生或发生极其微弱的界面反应。
c. 所制备的Fe/Si3N4复合粒子结构是特殊“芯-壳”(core-shell)微结构,即Si3N4芯核分别被连续、很薄的SiOxNy,SiO2和Fe壳体层层包裹,层与层之间化学键结合紧密。
参考文献:
[1] 史晓亮, 杨 华, 邵刚勤, 等. Al2O3/WC-10Co/ZrO2/Ni金属陶瓷的微波烧结[J]. 中南大学学报: 自然科学版, 2007, 38(4): 623-628.
SHI Xiao-liang, YANG Hua, SHAO Gang-qin, et al. Microwave sintering of Al2O3/WC-10Co/ZrO2/Ni cermets[J]. Journal of Central South University: Science and Technology, 2007, 38(4): 623-628.
[2] 刘 勋, 范景莲, 凌国纳. 纳米Ni-Al2O3金属陶瓷粉末热压致密化过程[J]. 中南大学学报: 自然科学版, 2004, 35(1): 21-25.
LIU Xun, FAN Jing-lian, LING Guo-na. Hot pressing densification process for nano Ni-Al2O3 powders[J]. Journal of Central South University: Science and Technology, 2004, 35(1): 21-25.
[3] 陈俊红, 孙加林, 洪彦若, 等. 铁元素在氮化硅铁中的存在状态[J]. 硅酸盐学报, 2004, 32(11): 1347-1351.
CHEN Jun-hong, SUN Jia-lin, HONG Yan-ruo, et al. Distribution status of Fe in ferro silicon nitride[J]. Journal of The Chinese Ceramic Society, 2004, 32(11): 1347-1351.
[4] 陈俊红, 孙加林, 占华生, 等. 较低温度下制备自结合氮化硅铁制品[J]. 北京科技大学学报, 2005, 27(5): 586-588.
CHEN Jun-hong, SUN Jia-lin, ZHANG Hua-rong, et al. Preparation of self-bonded products on the base of Fe-Si3N4 at low temperature[J]. Journal of University of Science and Technology Beijing, 2005, 27(5): 586-588.
[5] 王林俊. MgO-Si3N4复合耐火材料制备、结构、组成和性能研究[D]. 北京: 北京科技大学材料科学与工程学报, 2004.
WANG Lin-jun. Study on preparation process and compositions, structure and properties of MgO/Si3N4 composite refractory[D]. Beijing: School of Materials Science and Engineering, University of Science and Technology Beijing, 2004.
[6] CHEN Jun-hong, WANG Xue-da, SUN Jia-lin, et al. Research on the Fe-silicon nitride material self-producing N2 at high temperature[J]. Journal of University of Science and Technology Beijing, 2006, 13(1): 78-81.
[7] Fh J D, Bressiani J C. Effect of iron and silicon addition on the densification, microstructure and mechanical properties of silicon nitride[J]. Materials Science and Engineering, 1996, A209: 164-168.
[8] Heikinheimo E, Isomaki I, Kodentsov A A, et al. Chemical interaction between Fe and silicon nitride ceramic[J]. Journal of European Ceramic Society, 1997(17): 25-31.
[9] Hideki K, Takene H, Tateoki I. Frictional and mechanical properties of Fe5Si3-particle-dispersed Si3N4 fabricated by solution-infiltration method[J]. Journal of the Ceramic Society of Japan, 2003(1291): 193-199.
[10] Junichi T, Katsutoshi K, Takeshi M, et al. Effects of process parameters on post reaction sintering of silicon nitride ceramics[C]//Novel Processing of Ceramics and Composites- Proceedings of the 6th Pacific Rim Conference on Ceramic and Glass Technology. Maui: American Ceramic Society, 2006.
[11] Fate W A, Milberg M E. Effects of Fe and FeSi2 on the nitriding of Si powder[J]. Journal of the American Ceramic Society, 1978, 61(11/12): 531-532.
[12] 张 锐, 高 濂, 虞 玲, 等. Cu颗粒包覆纳米SiC粉体的相分散性能分析[J]. 无机材料学报, 2002, 17(5): 1059-1062.
ZHANG Rui, GAO Lian, YU Ling, et al. Analysis on the phase distribution of nano SiC particles coated with Cu[J]. Journal of Inorganic Materials, 2002, 17(5): 1059-1062.
[13] Kishimoto S, Shinya N. Development of metallic closed cellular materials containing polymers[J]. Mater Des, 2000, 21(6): 575-578.
[14] SHEN Xiang-qian, JING Mao-xiang, LI Dong-hong, et al. Fabrication of Fe, Ni and FeNi coated Al2O3 core-shell microspheres by the heterogeneous precipitation[J]. Powder Technology, 2005, 160(3): 229-233.
[15] 景茂祥, 沈湘黔, 李东红, 等. 金属Ni, FeNi包裹氧化铝复合微粉制备过程[J]. 江苏大学学报: 自然科学版, 2005, 26(6): 501-505.
JING Mao-xiang, SHEN Xiang-qian, LI Dong-hong, et al. Preparation of Ni and FeNi-coated Al2O3 composite micro-powders[J]. Journal of Jiangsu University: National Science Edition, 2005, 26(6): 501-505.
[16] 洪班德, 崔约贤. 材料电子显微分析实验技术[M]. 哈尔滨: 哈尔滨工业大学出版社, 1990.
HONG Ban-de, CUI Yue-xian. Electron microscopy technique of materials[M]. Harbin: Harbin Institute of Technology Press, 1990.
[17] 徐常明, 王士维, 黄校先, 等. 无压烧结制备Si3N4/SiO2复合材料[J]. 无机材料学报, 2006, 21(4): 935-938.
XU Chang-ming, WANG Shi-wei, HUANG Xiao-xian, et al. Preparation of Si3N4/SiO2 composites with pressureless sintering [J]. Journal of Inorganic Materials, 2006, 21(4): 935-938.
[18] QI Gong-jin, ZHANG Chang-rui, HU Hai-feng. Effects of precoating and calcination on microstructure of 3D silica fiber reinforced silicon nitride based composites[J]. Transactions of Nonferrous Metals Society of China, 2006, 16(4): 824-827.
[19] Ogbuji L U J T. The SiO2-Si3N4 interface: Ⅰ. Nature of the interphase[J]. J Am Ceram Soc, 1995, 78(5): 1272-1278.
[20] Ogbuji L U J T. The SiO2-Si3N4 interface: Ⅱ. O2 permeation and oxidation reaction[J]. J Am Ceram Soc, 1995, 78(5): 1279-1284.
[21] 姚红英, 顾 晓, 季 敏, 等. SiO2-羟基表面上金属原子的第一性原理研究[J]. 物理学报, 2006, 55(11): 6042-6046.
YAO Hong-ying, GU Xiao, JI Min. et al. First-principles study of metal atoms adsorbed on SiO2 surface[J]. Acta Physica Sinica, 2006, 55(11): 6042-6046.
收稿日期:2007-05-10;修回日期:2007-07-02
基金项目:国家自然科学基金委员会创新研究群体科学基金资助项目(50721003);教育部新世纪人才计划项目(NCET-05-0693)
作者简介:银锐明(1973-),男,湖南邵阳人,高级工程师,从事金属陶瓷材料研究
通信作者:范景莲,女,教授;电话:0731-8836652;E-mail: fjl@mail.csu.edu.cn


