Trans. Nonferrous Met. Soc. China 24(2014) 3716-3721
Preparation of electronic grade manganese sulfate from leaching solution of ferromanganese slag
Sheng YAN1,2, Yun-ren QIU1
1. School of Chemistry and Chemical Engineering, Central South University, Changsha 410083, China;
2. Innovation Base of Energy and Chemical Materials for Graduate Students Training of Hunan Province, Changsha 410083, China
Received 11 November 2013; accepted 6 June 2014
Abstract:
Preparation of electronic grade manganese sulfate from ferromanganese slag, including grinding, leaching and purification, was studied. The impurities, such as Fe3+, Al3+, Ca2+, Mg2+, heavy metal ions and Na+, K+, were removed from leaching solution by neutralized-hydrolysis, fluorination precipitation, sulfuration precipitation and re-crystallization. Effects of pH of reaction, temperature and dosage of the different additives on removal rates of the metallic ions in leaching solution were investigated, and the suitable temperature, pH and the added amount of precipitating agent were obtained. The prepared manganese sulfate product, of which the mass fractions of Ca2+, Mg2+, Na+, K+ are all smaller than 0.005%, the mass fractions of Fe3+, Al3+ and heavy metal ions are smaller than 0.001%, and the mass fraction of Mn2+ is greater than 32%, can meet the demand of anode materials of lithium-ion batteries.
Key words:
ferromanganese slag; leaching; purification; manganese sulfate; utilization of solid waste;
1 Introduction
With the development of lithium-ion battery industry, the electronic grade manganese sulfate for anode material of lithium-ion batteries is widely used in the production of new generation of environmentally friendly high-energy batteries [1-3].
Ferromanganese slag is a kind of blast furnace manganese slag from ferromanganese alloy smelting, a great amount of ferromanganese slag accounted for about 2-3 times as much as ferromanganese alloy each year [4,5]. The impurity categories included in ferro- manganese slag are the same as those of manganese ore, and the content of MnO is higher than that of ordinary manganese slag. Besides, there are no elements which have influence on the battery performance expect calcium, magnesium, iron, aluminum, potassium, sodium and heavy metal. However, these impurities can be removed by different purification technologies in order.
The preparation of electronic grade manganese sulfate from ferromanganese slag can lower the production cost of electronic grade manganese sulfate, bringing economic benefits and avoiding the environment pollution. WANG [6] reported that manganese was obtained through reduction method using sulfur dioxide as reducing agent and sintering method with ferromanganese slag as the raw material. SAFARIAN et al [7] studied the kinetics and mechanism of the simultaneous carbothermic reduction of FeO and MnO from high-carbon ferromanganese slag. However, no studies have been reported on the preparation of electronic grade manganese sulfate from ferromanganese slag due to it containing many impurities, such as iron, aluminum, potassium, sodium, calcium and magnesium, which makes it difficult to reduce the mass concentrations of Na+, K+, Ca2+ and Mg2+ smaller than 0.005%, and that of heavy metal is smaller than 0.001%.
In this work, preparation of electronic grade manganese sulfate from ferromanganese slag, including leaching and purification, was first studied, and the suitable condition was obtained. It can give guidance to the industrial production of the electronic grade manganese sulfate from ferromanganese slag.
2 Experimental
2.1 Material and analysis method
Manganese fluoride (MnF2) and sulfuric acid (H2SO4) were of industrial grade, sodium sulfide (Na2S) and manganese carbonate (MnCO3) were of analytical purity. Ferromanganese slag used in the experiment was came from a smelter in Hunan, which was analyzed by XRD and the results were listed in Table 1. Mass concentrations of various ions were analyzed by an inductively coupled plasma-atomic emission spectrometer (ICP-AES)
Table 1 Major composition of ferromanganese slag (mass fraction, %)

2.2 Experimental procedure
The preparation of electronic grade manganese sulfate from ferromanganese slag is described as follows. 200 g of ferromanganese slag powder ground to 250 μm or less was added to 700 mL of 30% H2SO4 solution, and reacted for 3 h at 80 °C under stirring. The leaching solution with mass concentration of Mn2+ of about 35 g/L, was obtained by filtration. Solid MnCO3 was added to the leaching solution to adjust pH so that the hydroxide precipitation of Fe3+ and Al3+ could be formed, and the precipitated hydroxides were removed by filtration. The obtained filtrate was evaporated till mass concentration of Mn2+ arrived at 92-94 g/L. Then, a certain amount of solid MnF2 was added to the concentrated solution so that the fluoride precipitation of Ca2+ and Mg2+ could be formed, and the precipitated fluorides were removed by filtration. And then, a certain amount of solid Na2S was added to the filtrate so that sulfide precipitation of heavy metal ions could be formed, and the precipitated sulfides were removed by filtration. Finally, the filtrate was evaporated, the crystal of MnSO4 was obtained by filtration, then washed with deionized water. In order to further reduce the mass fraction of Na+ and K+, the obtained crystal was re-dissolved, and the target product was obtained by re-crystallization and drying. The flow chart is shown in Fig. 1.
3 Results and discussion
3.1 Removal of iron, aluminum and silicon ions
Iron, aluminum and silicon ions are the main associated elements of ferromanganese slag, so iron, aluminum and silicon ions should be removed at first. Elimination of iron and aluminum ions can be obtained by adjusting a certain pH and temperature to form the colloids of Fe3+ and Al3+ as Fe(OH)3 and Al(OH)3, respectively, and the colloid can adsorb silicate in leaching solution, while Mn2+ remains in solution.
Figure 2 exhibits the effect of temperature of reaction on mass concentration of silicon in filtrate under the conditions of stirring rate of 500 r/min, pH 5.5. With the increase of temperature from 20 to 95 °C, the mass concentration of silicon in filtrate continuously decreases. When the reaction temperature is greater than 80 °C, the mass concentration of silicon is smaller than 2 mg/L in filtrate, which can meet the production of electronic grade manganese sulfate. So the optimal temperature of reaction is 80-95 °C.
However, the pH needs to be controlled strictly to avoid the manganese loss due to precipitation of manganese hydroxide [7,8]. Figure 3 exhibits the effect of pH on mass concentration of Fe3+ and Al3+ in filtrate. The leaching solution was treated by adding solid MnCO3 to adjust pH from 4 to 6 at 80-90 °C for 1.5 h.
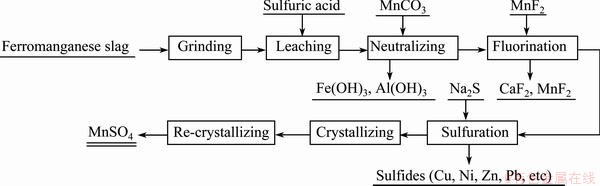
Fig. 1 Flow chart of preparation of electronic grade manganese sulfate
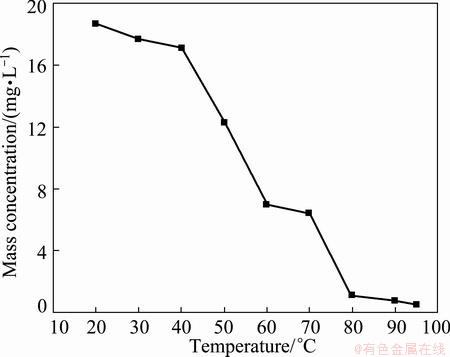
Fig. 2 Effect of temperature on mass concentration of silicon
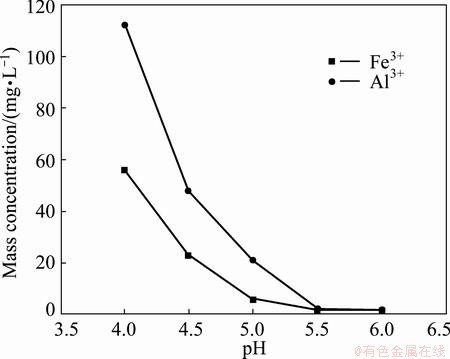
Fig. 3 Effect of pH on mass concentrations of Fe3+ and Al3+ in filtrate
The equation of metal hydroxide precipitation can be expressed as follows:
Mn++nOH- M(OH)n (1)
M(OH)n (1)
The equilibrium constant can be written as
 (2)
(2)
where Ks is the solubility product, and M is Fe, Al or Mn.
Equation (2) can be written as
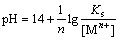 (3)
(3)
According to Eq. (3) and the practical concentrations of Fe3+, Al3+ and Mn2+ in leaching solution, the pH values of the starting precipitation of Fe3+, Al3+ and Mn2+ are 1.6, 2.8 and 6.5, respectively. Therefore, the pH is not greater than 6.5. Figure 3 shows that with the increase of the pH from 4 to 6, the mass concentration of Fe3+ decreases from 56 mg/L to 1.6 mg/L and that of Al3+ decreases from 112 mg/L to 1.8 mg/L. When pH is greater than 5.5, the mass concentrations of Fe3+ and Al3+ are smaller than 2 mg/L, which can meet the production of electronic grade manganese sulfate. Thus, the suitable pH is 5.5-6.0.
However, it is very difficult to filtrate for the formation of Fe(OH)3 colloidal solution at room temperature. When the temperature is greater than 80 °C, the precipitation of Fe(OH)3 is easily separated by filtration [9]. Thus, the suitable temperature is 80-90 °C.
3.2 Removal of calcium and magnesium ions
Generally, it gets difficult to remove calcium and magnesium ions from the solution containing manganese, because the chemical properties of Ca2+ and Mg2+ are similar to those of Mn2+ [10]. The different technologies of purification such as crystallization at high temperatures, solvent extraction and adsorption onto active carbon can remove about 90 % of Ca2+ and Mg2+ from manganese sulfate solution, but the mass concentrations of Ca2+ and Mg2+ in solution cannot meet the demand of the anode material of lithium-ion batteries. Thus, a suitable way needs to be found to effectively remove calcium and magnesium impurities from the solution containing manganese.
The filtrate, of which Fe3+ and Al3+ had been removed, was treated by adding solid MnF2 and controlling the reaction condition to remove Ca2+ and Mg2+. Figure 4 exhibits the effect of pH of fluorination reaction on mass concentrations of Ca2+ and Mg2+ in filtrate under the condition of adding 1.3 times the theoretical amount of MnF2, temperature 90 °C and time 1.5 h. With the increase of pH from 4.0 to 6.5, the mass concentration of Ca2+ decreases from 88 mg/L to 7 mg/L, and that of Mg2+ decreases from 96 mg/L to 10 mg/L. When the pH is smaller than 4, the precipitations of Ca2+ and Mg2+ as CaF2 and MgF2 respectively are not complete because F- reacts with H+ to form HF and its dissociation constant (KHF=7.4×10-4) is relatively small [11]. When the pH is greater than 6, the mass concentrations of Ca2+ and Mg2+ are smaller than 14 mg/L in filtrate. But the excessively high pH could cause the loss of manganese. Thus, the optimal pH of fluorination reaction is 6.
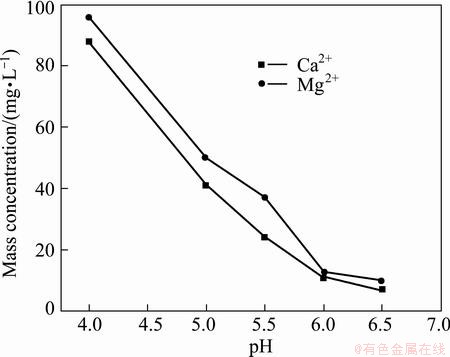
Fig. 4 Effect of pH of fluorination reaction on mass concentrations of Ca2+ and Mg2+ in filtrate
Figure 5 exhibits the effect of fluorination reaction temperature on mass concentrations of Ca2+ and Mg2+ in filtrate under the condition of adding 1.3 times the theoretical amount of MnF2, pH 6 and time 1.5 h. With the increase of fluorination reaction temperature from 60 °C to 95 °C, the mass concentration of Ca2+ decreases from 74 mg/L to 5 mg/L and Mg2+ from 92 mg/L to 8 mg/L, because the fluorination reactions are endothermic, heating is conducive to the generation of CaF2 and MgF2, and CaF2 and MgF2 have superior filter performance at higher temperatures [12,13]. When the fluorination reaction temperature is greater than 90 °C, the mass concentrations of Ca2+ and Mg2+ are smaller than 14 mg/L in filtrate, which can meet the production of electronic grade manganese sulfate. Thus, the optimal fluorination temperature is 90-95 °C.
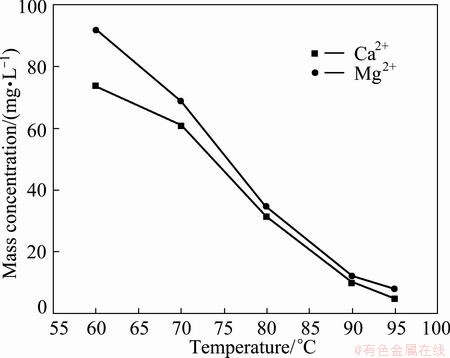
Fig. 5 Effect of fluorination temperature on mass concentrations of Ca2+ and Mg2+ in filtrate
Figure 6 exhibits the effect of the added amount of MnF2 on mass concentrations of Ca2+ and Mg2+ in filtrate under the condition of the pH 6.5, temperature 90 °C and time 1.5 h, where nMnF2 is the mass ratio of practical amount of MnF2 to theoretical amount by reaction equation. When nMnF2 is smaller than 1.2, the precipitations of Ca2+ and Mg2+ as CaF2 and MgF2 respectively are not complete due to the lack of F-. When nMnF2 is 1.2-1.4, the mass concentrations of Ca2+ and Mg2+ are smaller than 14 mg/L, which can meet the production of electronic grade manganese sulfate. While, nMnF2 is greater than 1.2, and the mass concentrations of Ca2+ and Mg2+ do not decrease. Thus, 1.2 times the theoretical amount of MnF2 is suitable.
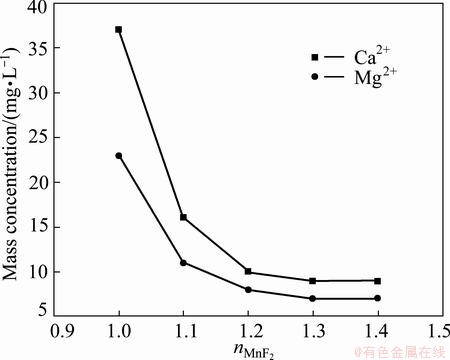
Fig. 6 Effect of added amount of MnF2 on mass concentrations of Ca2+ and Mg2+ in filtrate
3.3 Removal of heavy metal ions
The filtrate, of which Ca2+ and Mg2+ had been removed, was treated by adding solid Na2S to remove heavy metal ions based on the different sulfide solubilities of metals at a certain condition [14, 15]. The effect of pH on mass concentrations of heavy metal ions in filtrate is illustrated using Ni2+ as example because the solubility product of NiS is larger than that of other heavy metal sulfides such as CuS, CdS, PbS, ZnS and CoS. Figure 7 exhibits the effect of pH on mass concentration of Ni2+ in filtrate by adding 1.4 times the theoretical amount of Na2S during reaction of 1.5 h at 90 °C.
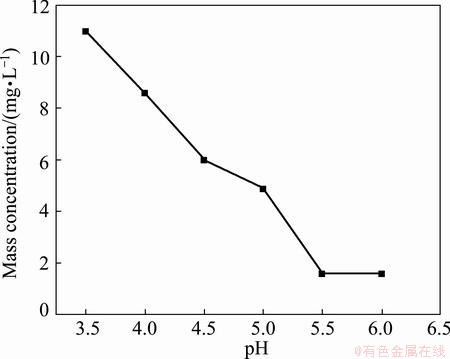
Fig. 7 Effect of pH of sulphuration reaction on mass concentration of Ni2+ in filtrate
The thermodynamic equilibrium involved in the sulfide precipitation can be expressed as follows [16,17]:
M2Sn 2Mn++nS2-, Ksp=[Mn+]2[S2-]n (4)
2Mn++nS2-, Ksp=[Mn+]2[S2-]n (4)
H2S(aq)  H++HS-, K1=10-8 (5)
H++HS-, K1=10-8 (5)
HS- H++S2-, K2=10-12.9 (6)
H++S2-, K2=10-12.9 (6)
H2S(aq)  2H++S2-,
2H++S2-,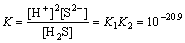 (7)
(7)
where Ksp is the solubility product of metals sulfide; K, K1 and K2 are dissociation equilibrium constants. These relationships can be written as
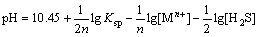 (8)
(8)
According to Eq. (8), the pH of fluorination reaction relates to not only the solubility product of metal sulfides, but also the practical mass concentration of metal ions. The mass concentration of manganese is relatively greater than that of other heavy metal ions in solution, and the excessively high pH may cause the loss of manganese. Figure 7 shows that the mass concentration of Ni2+ decreases from 11 mg/L to 1.6 mg/L with the increase of pH from 3.5 to 6.0. When the pH is greater than 5.5, the mass concentration of Ni2+ is smaller than 2 mg/L, which can meet the production of electronic grade manganese sulfate. Thus, the optimal pH of fluorination is 5.5-6.0.
Figure 8 exhibits the effect of the added amount of Na2S on the mass concentration of heavy metal ions in filtrate after reaction for 1.5 h under 90 °C and pH 6, where nNa2S is the mass ratio of practical amount of Na2S to theoretical amount by reaction equation. The mass concentration of Ni2+ decreases from 9 mg/L to 1.4 mg/L as nNa2S increases from 1.1 to 1.5. When nNa2S is smaller than 1.3, the precipitation of Ni2+ as NiS is not complete because a small part of S2- reacts with H+ to form H2S. When nNa2S is greater than 1.3, and the mass concentration of Ni2+ is smaller than 2 mg/L, which can meet the production of electronic grade manganese sulfate. Thus, 1.3 times the theoretical amount of Na2S is suitable.
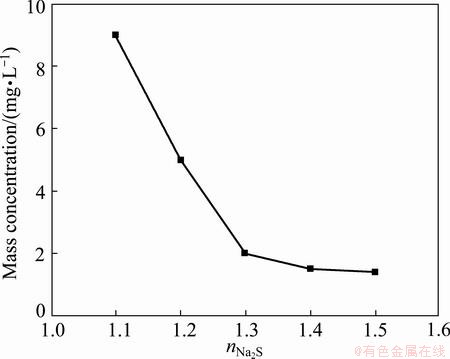
Fig. 8 Effect of added amount of Na2S on mass concentration of Ni2+ in filtrate
3.4 Removal of sodium and potassium ions
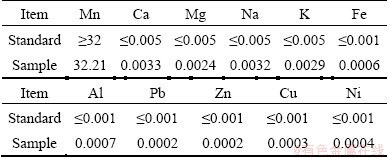
200 mL of filtrate, of which heavy metal ions had been removed and mass concentration of Mn2+ was 94 g/L, was evaporated till 10 mL or so under stirring at temperature from 95 °C to 100 °C; the concentrated suspension was filtered at 95-100 °C; the obtained crystal of MnSO4·H2O was washed with 95-100 °C deionized water and dried at 110 °C. The mass fractions of Na+ and K+ of the obtained product were greater than 0.005% (see Table 2). Thus, the obtained product was dissolved again with deionized water, the obtained solution was evaporated till 5 mL, the suspension was filtered at 95-100 °C and the obtained crystal of MnSO4·H2O was washed and dried at 110 °C. 51 g of MnSO4·H2O was obtained, and the recovery rate of manganese was 88%. The mass fractions of Na+ and K+ greatly decreased. The mass fractions of Na+ and K+ before and after re-crystallization are shown in Table 2, and the quality of prepared sample is shown in Table 3.
Table 2 Mass fractions of Na+ and K+

Table 3 Enterprise standard of electronic grade manganese sulfate (mass fraction, %)
4 Conclusions
1) Iron and aluminum ions in the leaching solution are removed with neutralization-hydrolysis by adjusting pH using solid MnCO3, under suitable conditions of pH 5.5-6.0, temperature 85-90 °C and time 1.5 h. The mass concentrations of Fe3+ and Al3+ are smaller than 2 mg/L.
2) Calcium and magnesium ions in the leaching solution are removed with fluoride precipitation by adding solid MnF2, under suitable conditions of 1.2 times theoretical amount of MnF2, pH 6, temperature 90-95 °C and time 1.5 h. The mass concentrations of Ca2+ and Mg2+ are smaller than 14 mg/L.
3) Heavy metal ions are removed with sulfide precipitation by adding solid Na2S, under suitable conditions of 1.3 times theoretical amount of Na2S, pH 5.5-6.0, temperature 90-95 °C and time 1.5 h. The mass concentration of heavy metal ions is smaller than 2 mg/L.
4) Sodium and potassium ions are removed with re-crystallization at temperature of 95-100 °C, and the mass fractions of Na+ and K+ are all smaller than 0.005%.
References
[1] MANTHIRAM A. Materials challenges and opportunities of lithium ion batteries [J]. The Journal of Physical Chemistry Letters, 2011, 2(3): 176-184.
[2] MURALIGANTH T, STROUKOFF K R, MANTHIRAM A. Microwave-solvothermal synthesis of nanostructured Li2MSiO4/C (M=Mn and Fe) cathodes for lithium-ion batteries [J]. Chemistry of Materials, 2010, 22(20): 5754-5761.
[3] HU Guo-rong, ZHANG Xin-long, PENG Zhong-dong. Preparation and electrochemical performance of tantalum-doped lithium titanate as anode material for lithium-ion battery [J]. Transactions of Nonferrous Metals Society of China, 2011, 21(10): 2248-2253.
[4] ETACHERI V, MAROM R, ElAZARI R, SALITRA G, AURBACH D. Challenges in the development of advanced Li-ion batteries: A review [J]. Energy & Environmental Science, 2011, 4: 3243-3262.
[5] YANG Zhi-zhong. The current situation and developing trend of ferro-alloy of Mn-series in China [J]. China’s Manganese Industry, 2005, 23(4): 1-6. (in Chinese)
[6] WANG Kun, CHEN Bin. Reusing and application of ferromanganese slag [J]. Environmental Engineering, 2003, 21(6): 53-55. (in Chinese)
[7] SAFARIAN J, KOLBEINSEN L, TANGSTAD M, TRANELL G. Kinetics and mechanism of the simultaneous carbothermic reduction of FeO and MnO from high-carbon ferromanganese slag [J]. Metallurgical and Materials Transactions B, 2009, 40(6): 929-939.
[8] VEGLIO F, TORO L. Fractional factorial experiments in the development of manganese dioxide leaching by sucrose in sulphuric acid solutions [J]. Hydrometallurgy, 1994, 36(2): 215-230.
[9] PAGNANELLI F, GARAVINI M, VEGLIO F, TORO L. Preliminary screening of purification processes of liquor leach solutions obtained from reductive leaching of low-grade manganese ores [J]. Hydrometallurgy, 2004, 71(3-4): 319-327.
[10] DUTRIZAC J E. Factors affecting alkali jarosite precipitation [J]. Metallurgical Transactions B, 1983, 14(4): 531-539.
[11] SAFAEEFAR P, ANG H M, KURAMOCHI H, ASAKUMA Y, MAEDA K, TADE M O, FUKUI K. Measurement and correlation of the solubility of MnSO4·H2O in ethanol + water + MgSO4· 7H2O solutions [J]. Fluid Phase Equilibria, 2006, 250(1-2): 64-69.
[12] LIU Hong-gang, ZHU Guo-cai. Removal of Ca(II), Mg(II) from leaching solution of low-grade manganese ore by precipitation with fluoride [J]. Mining & Metallurgy, 2007, 16(4): 25-28.
[13] BAKHSHESHI-RAD H R, IDRIS M H, KADIR M R A, DAROONPARVAR M. Effect of fluoride treatment on corrosion behavior of Mg-Ca binary alloy for implant application [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(3): 699-710.
[14] XIONG Li-zhi, CHEN Qi-yuan, YIN Zhou-lan, ZHANG Ping-min, DING Zhi-ying, LIU Zhi-xiong. Preparation of metal zinc from hemimorphite by vacuum carbothermic reduction with CaF2 as catalyst [J]. Transactions of Nonferrous Metals Society of China, 2012, 22(3): 694-699.
[15] JANDOVA J, LISA K, VU H,VRANKA F. Separation of copper and cobalt-nickel sulfide concentrates during processing of manganese deep ocean nodules [J]. Hydrometallurgy, 2005, 77(1-2): 75-79.
[16] LIANG Sha, GUO Xue-yi, FENG Ning-chuan, TIAN Qing-hua. Effective removal of heavy metals from aqueous solutions by orange peel xanthate [J]. Transactions of Nonferrous Metals Society of China, 2010, 20(1): 187-191.
[17] OKTAYBAS C, ACMA E, ARSIAN C, ADDEMIR O. Kinetics of copper precipitation by H2S from sulfate solutions [J]. Hydrometallurgy, 1994, 35(1): 129-137.
锰铁合金渣浸出液制备电子级硫酸锰
闫 升1,2,邱运仁1
1. 中南大学 化学化工学院,长沙 410083;
2. 湖南省能源与化工新材料研究生培养创新基地,长沙 410083
摘 要:以锰铁合金渣为原料,经研磨、硫酸浸出后,采用中和-水解、氟化沉淀、硫化沉淀和重结晶法去除浸出液中的铁、铝、钙、镁和重金属以及钠、钾等离子以制备电子级硫酸锰。研究反应pH、反应温度和不同添加剂的用量对硫酸锰产品中杂质含量的影响,并得到了较优的温度、pH、沉淀剂的用量等工艺条件。结果表明:在较优工艺条件下,所制备的碳酸锰中,Ca2+、Mg2+、Na+、K+杂质离子的含量小于0.005 %,Fe3+、Al3+和重金属离子的含量小于0.001%,Mn2+的含量大于32%,硫酸锰产品的质量满足锂离子电池正极材料的要求。
关键词:锰铁合金渣;浸出;纯化;硫酸锰;固废利用
(Edited by Xiang-qun LI)
Foundation item: Project (2013ZX0754-001) supported by China National Critical Project for Science and Technology on Water Pollution Prevention and Control
Corresponding author: Yun-ren QIU; Tel: +86-731-88879616; E-mail: qiuyunren@gmail.com; csu_tian@csu.edu.cn
DOI: 10.1016/S1003-6326(14)63520-2
Abstract: Preparation of electronic grade manganese sulfate from ferromanganese slag, including grinding, leaching and purification, was studied. The impurities, such as Fe3+, Al3+, Ca2+, Mg2+, heavy metal ions and Na+, K+, were removed from leaching solution by neutralized-hydrolysis, fluorination precipitation, sulfuration precipitation and re-crystallization. Effects of pH of reaction, temperature and dosage of the different additives on removal rates of the metallic ions in leaching solution were investigated, and the suitable temperature, pH and the added amount of precipitating agent were obtained. The prepared manganese sulfate product, of which the mass fractions of Ca2+, Mg2+, Na+, K+ are all smaller than 0.005%, the mass fractions of Fe3+, Al3+ and heavy metal ions are smaller than 0.001%, and the mass fraction of Mn2+ is greater than 32%, can meet the demand of anode materials of lithium-ion batteries.


