Trans. Nonferrous Met. Soc. China 29(2019) 51-58
Microstructures and mechanical properties of AZ91D/0Cr19Ni9 bimetal composite prepared by liquid-solid compound casting
Jian-hua ZHAO1,2, Wen-qun ZHAO2, Shen QU2, Yan-qing ZHANG2
1. State Key Laboratory of Mechanical Transmission, Chongqing University, Chongqing 400044, China;
2. College of Materials Science and Engineering, Chongqing University, Chongqing 400044, China
Received 24 January 2018; accepted 12 November 2018
Abstract:
The liquid-solid compound casting technology was used to produce the AZ91D/0Cr19Ni9 bimetal composite without and with hot dipping aluminium, respectively. The influences of Al coating on microstructures and mechanical properties of AZ91D/0Cr19Ni9 interface were investigated. The results showed that the mechanical bonding was obtained between AZ91D and bare steel 0Cr19Ni9 where a gap existed at the interface; the metallurgical bonding was formed between AZ91D and Al-coated 0Cr19Ni9, which could be divided into two different intermetallic layers: layer I was mainly composed of α-Mg+β-Mg17Al12 eutectic structure and a small amount of MgAl2O4, and layer II mainly comprised of Fe2Al5 intermetallic compound. Furthermore, the hardness value of interface was obviously higher than that of AZ91D matrix, and the average hardness values of layers I and II were HV 158 and HV 493, respectively. The shear strength of AZ91D/Al-coated 0Cr19Ni9 interface was higher than that of AZ91D/bare 0Cr19Ni9 interface, which confirmed that Al coating could improve the adhesive strength between AZ91D and 0Cr19Ni9 during liquid-solid compound casting process.
Key words:
AZ91D; 0Cr19Ni9; liquid-solid compound casting; interface bonding; shear strength;
1 Introduction
Magnesium alloys have been considered as the promising structure materials due to low density, high specific strength, better specific stiffness, excellent castability, good noise and vibration damping capacity and favourable recycling capability [1-3]. These advantages of magnesium alloys make it possible for automobile manufacturers to lower vehicle mass and improve fuel efficiency. However, low hardness, low strength and poor corrosion resistance seriously limit the wide and further application of magnesium alloys. It is well-known that steel remains one of the dominant materials used in the automotive industry and has great high strength and excellent ductility. If the reliable joining between magnesium alloy and steel can be obtained by the liquid-solid compound casting, it would facilitate the utilization of magnesium alloys in the modern industry and provide a feasible method for further reducing the vehicle weight.
However, there are some challenges in joining dissimilar metals Mg and steel due to the great difference in their physical and mechanical properties. For example, the remarkable difference in the melting points between Mg (649 °C) and Fe (1539 °C) causes the difficulty in melting them at the same time via fusion welding. The maximum solid solubility of Fe in Mg is 0.00043 at.%, and solid solubility of Mg in Fe is zero [4]. Furthermore, both metals are immiscible at a liquid state, and they do not react with each other [5]. Currently, various techniques such as resistance spot welding [6,7], friction stir welding [8,9], laser weld-brazing [10,11], diffusion welding [5,12] and inert gas welding [13] {Wang, 2016 #8}have been used to join Mg and steel. Still, it is difficult to join magnesium alloys and steel by conventional fusion welding, leading to restrictions and limitations with these welding procedures due to their disadvantages in joining efficiency and flexibility.
Compound casting is characterized as a craft of joining two metals or alloys via pouring one liquid metal onto or around another solid metal. This craft has already been used to join a series of similar and dissimilar bimetallic materials such as Al/Al [14], Al/Cu [15,16], Al/Mg [17], steel/Al [18] and steel/Cu [19]. Nevertheless, there are few studies on joining magnesium to steel by this method. In this work, AZ91D/0Cr19Ni9 bimetal materials made of bare 0Cr19Ni9 were prepared by liquid-solid compound casting. For comparison, an Al coating was deposited on the 0Cr19Ni9 steel by hot dipping aluminium, then AZ91D/Al-coated 0Cr19Ni9 bimetal materials were also produced by liquid-solid compound casting. The microstructure and mechanical properties of bonding interface in both cases were analyzed systematically. The influence of Al coating on interfacial bonding of AZ91D/0Cr19Ni9 was discussed.
2 Experimental
2.1 Materials
AZ91D magnesium alloy was used as metal matrix materials. Rectangular 0Cr19Ni9 steel with dimensions of 30 mm × 100 mm × 2 mm was chosen as the embedding materials. The chemical compositions of AZ91D magnesium alloy and 0Cr19Ni9 steel are listed in Tables 1 and 2, respectively.
Table 1 Chemical composition of AZ91D Mg alloy (wt.%)

Table 2 Chemical composition of 0Cr19Ni9 steel (wt.%)
2.2 Preparation of AZ91D/0Cr19Ni9 composites
In order to get an excellent bonding between AZ91D magnesium alloy and 0Cr19Ni9 steel, surface treatments of 0Cr19Ni9 steel are essential. Firstly, the 0Cr19Ni9 insert was polished by sand paper and subjected to ultrasonic cleaning using acetone as a medium to remove the contamination on the surface. Subsequently, the treated 0Cr19Ni9 insert was immersed into the 10% ammonium chloride solution at 80 °C for 10 min and dried at 120 °C. Finally, the Al-coated 0Cr19Ni9 insert was placed inside the mould rapidly after dipping into the molten pure aluminum at 780 °C for 75 s. For comparison, the bare 0Cr19Ni9 insert was preheated at 250 °C and then placed in the mould rapidly for compound casting.
The AZ91D magnesium alloy ingot was melted under the protection of RJ2 covering flux in a crucible, which was placed in an electrical furnace. The metal mould was preheated at 250 °C. Before casting, the molten AZ91D magnesium alloy was refined. The molten AZ91D magnesium alloy was poured into the mould with the bare and the Al-coated 0Cr19Ni9 insert at 720 °C, respectively.
2.3 Characterization
The specimens of 10 mm × 10 mm × 10 mm were cut from the cross-section of the interface by computer numerical control line cutting machine and then polished by silicon carbide papers. After that, the 3 vol.% HNO3 in alcohol solution was used to etch the specimens. The microstructure and the major elements distributions of the etched specimens were analyzed by TESCAN VEGA 3 LMH scanning electron microscope (SEM) and X-ray spectroscopy (EDS) detector, respectively. The phase constitutions at the interface of AZ91D/0Cr19Ni9 were identified by D/max2500PC X-ray diffraction (XRD). An Everone MH-5L microhardness tester was used to measure the distributions of microhardness at the interface of AZ91D/0Cr19Ni9 under a testing load of 100 g and a holding time of 15 s. The shear strength of AZ91D/0Cr19Ni9 bimetallic materials was measured by a setup SANA which was described in our previous publication [20].
3 Results and discussion
3.1 Analysis of Al-coated 0Cr19Ni9
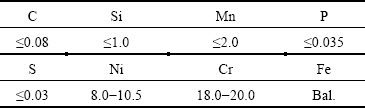
The SEM image and EDS analysis results of Al coating are shown from Fig. 1. It can be seen in Fig. 1(a) that two distinct reaction layers are obtained on 0Cr19Ni9 substrates after 15 s deposition: intermediate layer (Fe-Al layer) and Al coating, which are confirmed by the EDS map (as shown in Fig. 1(b)). The average thicknesses of Fe-Al layer and pure Al coating are about 17.4 and 24 μm, respectively, and the thickness of Fe-Al layer is relatively uniform. Moreover, the section of Fe-Al layer towards Al coating grows into dendrites, and Fig. 1(b) shows the distribution of major elements along the black line (as marked in Fig. 1(a)) from 0Cr19Ni9 steel to the Al coating. It reveals that Fe element decreases gradually; on the contrary, Al element rises gradually, and Cr and Ni elements diffuse slightly to Fe-Al layer. In Fe-Al layer, the content of Al element is obviously higher than that of Fe element, indicating that Al element diffuses quickly than Fe element, so the compound tends to be Al-rich phase. According to the energy spectrum analysis in Figs. 1(c) and (d), the Fe-Al intermetallic layer adjacent to 0Cr19Ni9 steel could be identified as Fe2Al5, while dendritic Fe-Al phase near to Al coating could be identified as FeAl3.
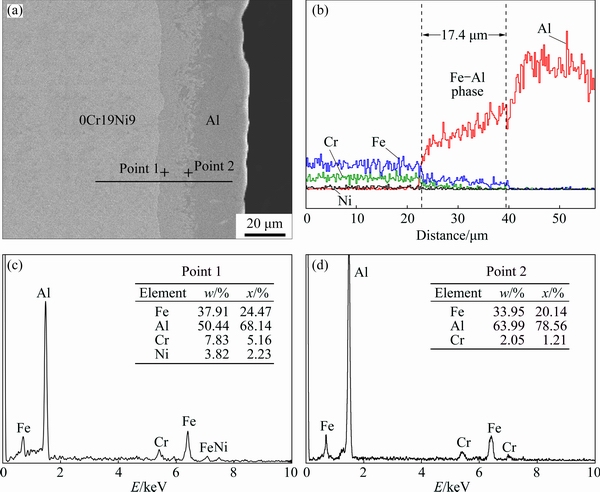
Fig. 1 SEM image of cross-section of Al-coated 0Cr19Ni9 (a), EDS line scan (b), and EDS spectra of points 1 (c) and 2 (d) marked in (a), respectively
3.2 Microstructural analysis and phase identification
Figure 2 presents the SEM micrograph and EDS line scan analysis across the interface zone of AZ91D/bare 0Cr19Ni9 and AZ91D/Al-coated 0Cr19Ni9, respectively. It could be observed from Fig. 2(a) that the AZ91D/0Cr19Ni9 interface zone has no interfacial layer, and there is a gap between AZ91D and bare 0Cr19Ni9. However, the interface of AZ91D/Al-coated 0Cr19Ni9 is continuous and homogeneous without evident defects and cracks seen in Fig. 2(c). Two different layers between AZ91D substrate and Al-coated 0Cr19Ni9 steel (I and II) could be seen by observing their colors and shapes. Layer I is next to the AZ91D substrate and layer II is next to the 0Cr19Ni9 steel, furthermore, the thicknesses of Layer I and layer II are approximately 19.6 and 20.6 μm, respectively. It is obvious that metallurgical bonding was obtained on the AZ91D/ Al-coated 0Cr19Ni9 interface. The results show that Al coating has great potential of contributing to the metallurgical bonding between magnesium and steel.
As shown in Fig. 2(b), from AZ91D to the gap, Mg content decreases sharply, and Al content has not changed too much substantially. A small peak of Mg content at 0Cr19Ni9 appeared near the gap. The contents of Fe and Cr increased significantly from gap to 0Cr19Ni9, while Ni content hardly changed. It is worth noticing that there was no reaction layer at the interface, suggesting that no atom diffusion or interfacial reaction occurred between AZ91D and bare 0Cr19Ni9. This result indicated that AZ91D and bare 0Cr19Ni9 were mechanical bonding via compound casting. As shown in Fig. 2(d), the content of Mg fluctuates from AZ91D substrate to layer I, which is contrary to that of Al. The main reason for this is that the increase of Al content is caused by the scan line crossing the precipitated Mg-Al phase. It is easy to see that the concentration of Mg was significant at the black phase where the Al content was very low, which indicated that layer I consisted of Mg-Al phase, Mg solid solution and a small amount of Al. The Mg content decreased sharply from layer I to layer II. However, the line scan result differed greatly regarding the variation of the Al content across the interface of AZ91D/bare 0Cr19Ni9. A high level Al content was observed in layer II. The Fe and Cr contents increased gradually from AZ91D to 0Cr19Ni9. In layer II, the mass ratio of Al to Fe was almost constant, indicating that layer II contained one Fe-Al intermetallic compound. Layer II was thicker than pre-existing Fe-Al layer (17.4 μm), indicating that atomic diffusion and chemical reaction occurred between Al and Fe and a new Fe-Al phase was formed.
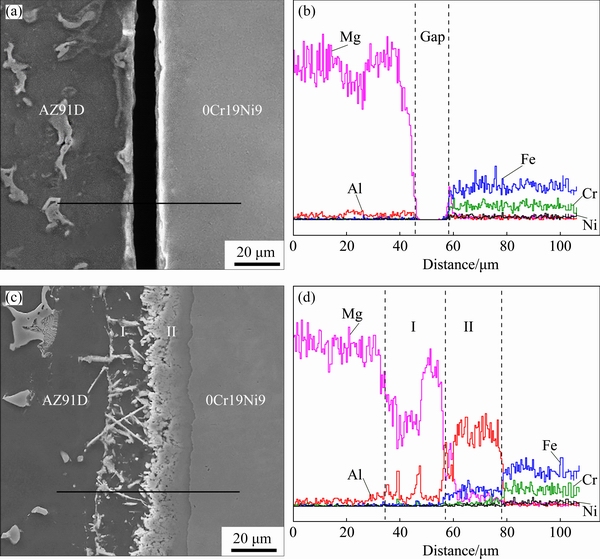
Fig. 2 SEM micrographs (a, c) and EDS line scans (b, d) of AZ91D/bare 0Cr19Ni9 interface (a, b) and of AZ91D/Al-coated 0Cr19Ni9 interface (c, d)
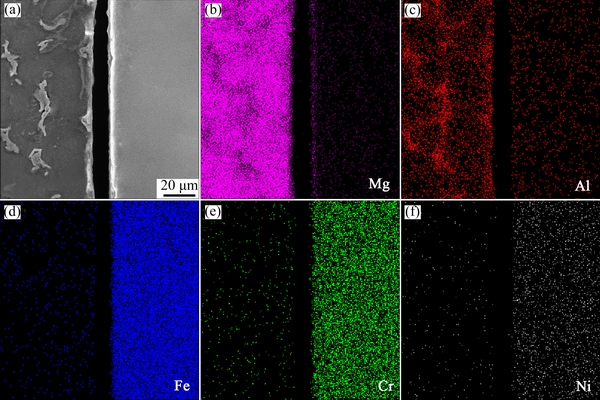
Fig. 3 SEM image (a) and elemental distributions (b-f) of AZ91D/bare 0Cr19Ni9 interface
The representative concentration profiles of Mg, Al, Fe, Cr and Ni across the interface between AZ91D and bare 0Cr19Ni9 are shown in Fig. 3. There is no intermetallic compound layer at the interface. As is well known, Mg and Fe do not react with each other [5]. AZ91D and bare 0Cr19Ni9 were mechanical bonding by compound casting.
The representative concentration profiles of Mg, Al, Fe, Cr and Ni across the interface between AZ91D and Al-coated 0Cr19Ni9 are shown in Fig. 4. Al was concentrated at the interface (corresponding to the observation in Fig. 2(d)), which was further proved by the result of EDS line scan. The results suggested that Mg-Al or Fe-Al intermetallic compounds were formed at the bonding interface during this process. Three kinds of phases could be distinguished clearly in layer I: white irregular block, needle-like phase, and the black substrate phase. What’s more, there was a single grey phase in layer II. The EDS analysis results of different points (points 1-5 in Fig. 4(a)) are listed in Table 3. It could be deduced that the white irregular block and needle-like phase were MgAl2O4 and Mg17Al12 in layer I, respectively, and the black substrate phase was α-Mg. In layer II, the single grey phase was Fe2Al5 phase.
The XRD pattern (shown in Fig. 5) of the interface in AZ91D/Al-coated 0Cr19Ni9 composite confirmed the existence of β-Mg17Al12 and Fe2Al5 intermetallic compounds at the interface. These results implied that layer I adjacent to AZ91D substrate mainly consisted of α-Mg solid solution, a small amount of needle-like β-Mg17Al12 phase and block MgAl2O4 phase. Layer II consisted of single Fe2Al5 phase.
3.3 Mechanical properties
Figure 6 shows Vickers microhardness distribution profiles across the interface. The Vickers microhardness distribution profile across the interface of AZ91D/bare 0Cr19Ni9 is illustrated in Fig. 6(a). The average microhardness values of AZ91D matrix and 0Cr19Ni9 steel were HV 67 and HV 196, respectively. Figure 6(b) shows the microhardness distribution profile across the interface of AZ91D/Al-coated 0Cr19Ni9. It was shown that the microhardness distribution at the interface was uneven. The hardness value of interface was higher than that of the AZ91D matrix, because intermetallic compounds with high hardness were formed at the interface. The average hardness value of layer I was HV 158 in virtual of formed Mg17Al12 phase. The hardness of layer II adjacent to 0Cr19Ni9 steel increased abruptly and reached the maximum value of HV 493, because layer II contained Fe2Al5 intermetallic compound.
Shear forces were applied to estimating the adhesive strength between AZ91D and 0Cr19Ni9. Table 4 displays the shear strength of AZ91D/0Cr19Ni9 interface. The shear strength of AZ91D/bare 0Cr19Ni9 interface was weak, which split during preparation of shear test samples by computer numerical control line cutting machine. The shear strength of AZ91D/Al-coated 0Cr19Ni9 interface was 26.8 MPa. The shear strength of AZ91D/Al-coated 0Cr19Ni9 interface was much higher than that of AZ91D/bare 0Cr19Ni9 interface. It can be convinced from the values that the Al coating is useful for improving the adhesive strength of AZ91D/0Cr19Ni9 bimetal materials by liquid-solid compound casting.
3.4 Bonding mechanism of AZ91D/Al-coated 0Cr19Ni9
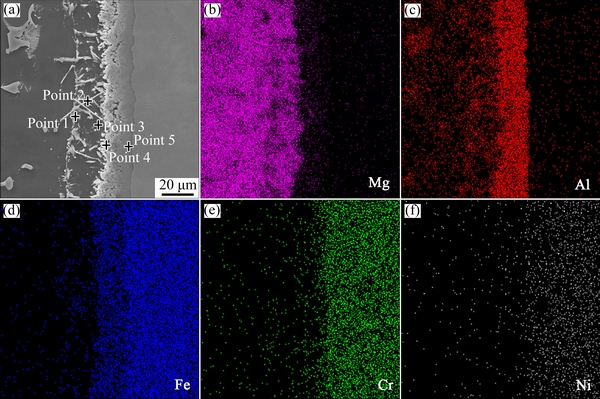
Fig. 4 SEM image (a) and elemental distributions (b-f) of AZ91D/Al-coated 0Cr19Ni9 interface
Table 3 Compositions of points 1-5 in Fig. 4(a) by EDS
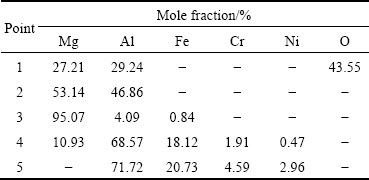
Fig. 5 XRD pattern of AZ91D/Al-coated 0Cr19Ni9 compound interface
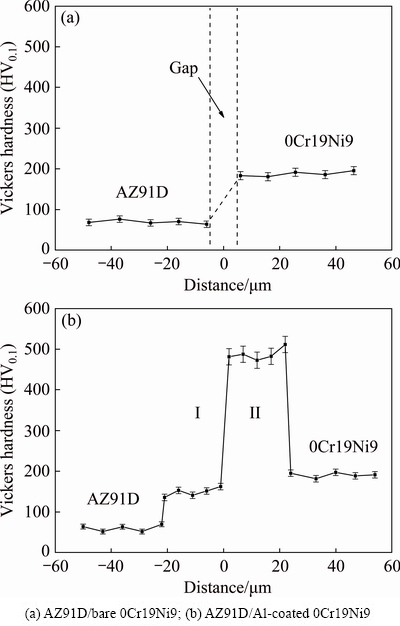
Fig. 6 Microhardness distribution profiles across interface
Table 4 Shear strength of AZ91D/0Cr19Ni9 interface

Based on the above analysis, the bonding mechanism of AZ91D to Al-coated 0Cr19Ni9 by liquid-solid compound casting could be elucidated. Figure 7 presents the schematic diagram of interfacial micro- structure evolution. Firstly, the Al-coated 0Cr19Ni9 steel was easy to form oxide film on the surface when it was exposed to the air. Therefore, the surface of Al-coated 0Cr19Ni9 steel was covered by aluminium oxide (Al2O3). Similarly, when the liquid AZ91D metal was poured into the mould, the liquid and the oxide film on the surface of Al-coated 0Cr19Ni9 steel contacted sufficiently. Furthermore, the molten magnesium could firstly react with the Al2O3 layer (on the surface of Al-coated 0Cr19Ni9 steel) to generate MgAl2O4 phase. MgAl2O4 phase was formed based on the following chemical equations:
3Mg+Al2O3→3MgO+2Al (1)
MgO+Al2O3→MgAl2O4 (2)
The layer of Al2O3 was disrupted owing to the formation of MgAl2O4. Then, Mg atoms diffused from AZ91D substrate into Al coating and reacted with Al element under the heating of molten AZ91D, as shown in Fig. 7(b). At the interface between AZ91D matrix and Al coating, there was a great concentration difference in Mg and Al. This is because the content of Mg was obviously higher than that of Al. According to diffusion theory, large numbers of Mg atoms diffused into Al coating because of the concentration gradient. When the concentration of the Mg atoms in the Al coating exceeded the solid solubility limit, the needle-like (α-Mg+β-Mg17Al12) eutectic structure was formed. According to the Mg-Al binary phase diagram, the (α-Mg+β-Mg17Al12) eutectic structure could be formed due to
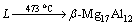 +α-Mg (3)
+α-Mg (3)
With Mg atoms diffusing into Al coating continuously, the Al coating decreased gradually. At the same time, the inter-diffusion and inter-reaction of Al from Al coating and Fe from the 0Cr19Ni9 steel at the interface resulted in the formation of Fe2Al5 phase (as shown in Fig. 7(c)). Additionally, the pre-existing Fe-Al phase was found to act as nucleation sites that accelerated the growth of newly formed Fe-Al layers at the interface. According to the Fe-Al binary phase diagram, Fe2Al5 phase could be generated following chemical equation below
L→Fe2Al5 (4)
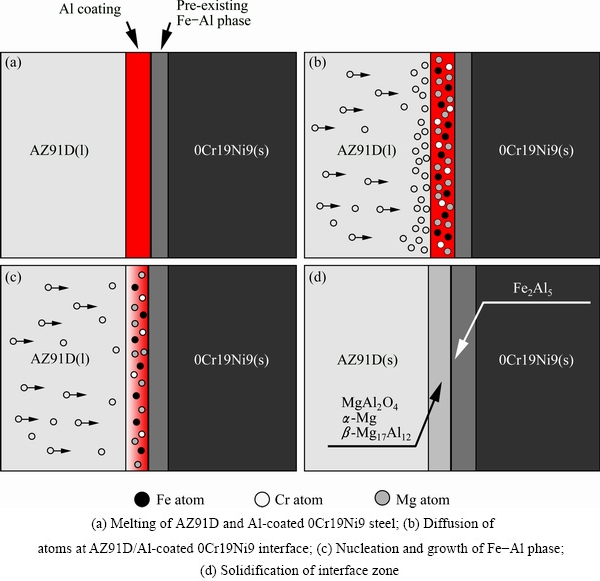
Fig. 7 Schematic illustration of interfacial reaction mechanism
In the liquid-solid compound casting process, diffusion, chemical reactions and solidification occurred at the interface at the same time. Therefore, a complex interface structure, which consisted of a small amount of MgAl2O4, the α-Mg+β-Mg17Al12 eutectic layer and the Fe2Al5 intermediate compound layer, was formed between the AZ91D and 0Cr19Ni9 interface, as shown in Fig. 7(d).
4 Conclusions
(1) Mechanical bonding was achieved between AZ91D and bare 0Cr19Ni9 by liquid-solid compound casting. There was a gap at the AZ91D/bare 0Cr19Ni9 interface.
(2) Metallurgical bonding was achieved between AZ91D and Al-coated 0Cr19Ni9 by liquid-solid compound casting. The AZ91D/Al-coated 0Cr19Ni9 interface zone was composed of two different layers. Layer I next to the AZ91D substrate mainly consisted of α-Mg, β-Mg17Al12 and a small amount of MgAl2O4. Layer II adjacent to the 0Cr19Ni9 steel was mainly composed of Fe2Al5 phase.
(3) The shear strength of AZ91D/Al-coated 0Cr19Ni9 interface was higher than that of AZ91D/bare 0Cr19Ni9 interface, which confirmed that Al coating could improve the adhesive strength between AZ91D matrix and 0Cr19Ni9 steel.
References
[1] KULEKCI M K. Magnesium and its alloys applications in automotive industry [J]. International Journal of Advanced Manufacturing Technology, 2007, 39: 851-865.
[2] YAO S, LI Y F. Review on the development and application of magnesium alloys [J]. Science of the Total Environment, 2015, 44(1): 89-96.
[3] TAN C W, CHEN B, SONG X G, ZHOU L, MENG S H, LI L Q, FENG J C. Influence of Al interlayer thickness on laser welding of Mg/steel [J]. Welding Journal, 2016, 95(S): s384-s394.
[4] BAKER H, OKAMOTO H. ASM handbook: Alloy phase diagrams [M]. Vol.3. Ohio: American Society for Metals, 1992: 151-153.
[5] LIU L M, ZHAO X. Study on the weld joint of Mg alloy and steel by laser-GTA hybrid welding [J]. Materials Characterization, 2008, 59(9): 1279-1284.
[6] FENG Yue-qiao, LI Yang, LUO Zhen, LING Zhan-xiang, WANG Zheng-ming. Resistance spot welding of Mg to electro-galvanized steel with hot-dip galvanized steel interlayer [J]. Journal of Materials Processing Technology, 2016, 236: 114-122.
[7] LIU L, XIAO L, CHEN D L, FENG J C, KIM S, ZHOU Y. Microstructure and fatigue properties of Mg-to-steel dissimilar resistance spot welds [J]. Materials & Design, 2013, 45: 336-342.
[8] SCHNEIDER C, WEINBERGER T, INOUE J, KOSEKI T, ENZINGER N. Characterisation of interface of steel/magnesium FSW [J]. Science & Technology of Welding & Joining, 2013, 16(1): 100-107.
[9] JANA S, HOVANSKI Y. Fatigue behaviour of magnesium to steel dissimilar friction stir lap joints [J]. Science & Technology of Welding & Joining, 2012, 17(2): 141-145.
[10] LI Li-qun, CHEN Yan-bin, GUO Wei, HU Xin-bin. Influence of Zn coating on interfacial reactions and mechanical properties during laser welding-brazing of Mg to steel [J]. Metallurgical & Materials Transactions A, 2012, 43(12): 4740-4754.
[11] TAN Cai-wang, LI Li-qun, CHEN Yan-bin, GUO Wei. Interfacial microstructure and fracture behavior of laser welded-brazed Mg alloys to Zn-coated steel [J]. International Journal of Advanced Manufacturing Technology, 2013, 68(5-8): 1179-1188.
[12] YUAN Xin-jian, SHENG Guang-min, LUO Jun, LI Jia. Microstructural characteristics of joint region during diffusion- brazing of magnesium alloy and stainless steel using pure copper interlayer [J]. Transactions of Nonferrous Metals Society of China, 2013, 23(3): 599-604.
[13] WANG Xiao-yong, SUN Da-qian, YIN Shi-qiang, LIU Dong-yang. Microstructures and mechanical properties of metal inert-gas arc welded Mg-steel dissimilar joints [J]. Transactions of Nonferrous Metals Society of China, 2015, 25(8): 2533-2542.
[14] FENG Jian, YE Bing, ZUO Li-jie, WANG Qu-dong, WANG Qi-gui, JIANG Hai-yan, DING Wen-jiang. Bonding of aluminum alloys in compound casting [J]. Metallurgical & Materials Transactions A, 2017, 48(10): 4632-4644.
[15] AKBARIFAR M, DIWANDARI M. On the interfacial characteristics of compound cast Al/brass bimetals [J]. International Journal of Metalcasting, 2016, 11(3): 1-7.
[16] HU Yuan, CHEN Yi-qing, LI Li, HU Huang-dong, ZHU Zi-ang. Microstructure and properties of Al/Cu bimetal in liquid–solid compound casting process [J]. Transactions of Nonferrous Metals Society of China, 2016, 26(6): 1555-1563.
[17] HAJJARI E, RAZAVI S H, EMAMI S M, HOMMA T, KAMADO S. Dissimilar joining of Al/Mg light metals by compound casting process [J]. Journal of Materials Science, 2011, 46(20): 6491-6499.
[18] JIANG Wen-ming, FAN Zi-tian, LI Chi. Improved steel/aluminum bonding in bimetallic castings by a compound casting process [J]. Journal of Materials Processing Technology, 2015, 226: 25-31.
[19] HO J S, LIN C B, LIU C H. Effect of continuous cooling heat treatment on interface characteristics of S45C/copper compound casting [J]. Journal of Materials Science, 2004, 39(7): 2473-2480.
[20] HE Ke, ZHAO Jian-hua, LI Pu, HE Jian-sheng, TANG Qi. Investigation on microstructures and properties of arc-sprayed-Al/ AZ91D bimetallic material by solid–liquid compound casting [J]. Materials & Design, 2016, 112: 553-564.
液-固复合铸造AZ91D/0Cr19Ni9双金属复合材料的显微组织和力学性能
赵建华1,2,赵文群2,屈 伸2,张彦青2
1.重庆大学 机械传动国家重点实验室,重庆 400044;
2.重庆大学 材料科学与工程学院,重庆 400044
摘 要:采用液-固复合铸造技术制备未经表面处理和经热浸镀铝表面处理的AZ91D/0Cr19Ni9双金属复合材料,研究铝涂层对AZ91D/0Cr19Ni9界面显微组织和力学性能的影响。结果表明:镁合金和裸钢0Cr19Ni9之间为机械结合,结合界面处存在一缝隙;而镁合金AZ91D和镀铝钢0Cr19Ni9之间形成冶金结合,且镁合金AZ91D/镀铝钢0Cr19Ni9界面可以分为两个不同的金属间化合物层:层I主要由α-Mg+β-Mg17Al12共晶组织和少量的MgAl2O4相组成,层II主要由Fe2Al5金属间化合物组成。此外,结合界面的硬度明显高于镁合金基体AZ91D的硬度,层I和层II的平均硬度分别为HV 158和HV 493。镁合金AZ91D/镀铝钢0Cr19Ni9界面的剪切强度高于镁合金 AZ91D/裸钢0Cr19Ni9界面的剪切强度,这证明在液-固复合铸造过程中铝涂层能提高镁合金AZ91D和钢0Cr19Ni9之间的结合强度。
关键词:AZ91D;0Cr19Ni9;液-固复合铸造;界面结合;剪切强度
(Edited by Wei-ping CHEN)
Foundation item: Project (cstc2015yykfC0001) supported by the National Engineering Research Centre for Magnesium Alloys, China; Project supported by State Key Laboratory of Mechanical Transmission of Chongqing University, China
Corresponding author: Jian-hua ZHAO; Tel/Fax: +86-23-65112611; E-mail: zjhwzf@sina.com
DOI: 10.1016/S1003-6326(18)64914-3
Abstract: The liquid-solid compound casting technology was used to produce the AZ91D/0Cr19Ni9 bimetal composite without and with hot dipping aluminium, respectively. The influences of Al coating on microstructures and mechanical properties of AZ91D/0Cr19Ni9 interface were investigated. The results showed that the mechanical bonding was obtained between AZ91D and bare steel 0Cr19Ni9 where a gap existed at the interface; the metallurgical bonding was formed between AZ91D and Al-coated 0Cr19Ni9, which could be divided into two different intermetallic layers: layer I was mainly composed of α-Mg+β-Mg17Al12 eutectic structure and a small amount of MgAl2O4, and layer II mainly comprised of Fe2Al5 intermetallic compound. Furthermore, the hardness value of interface was obviously higher than that of AZ91D matrix, and the average hardness values of layers I and II were HV 158 and HV 493, respectively. The shear strength of AZ91D/Al-coated 0Cr19Ni9 interface was higher than that of AZ91D/bare 0Cr19Ni9 interface, which confirmed that Al coating could improve the adhesive strength between AZ91D and 0Cr19Ni9 during liquid-solid compound casting process.


